Electronegativity: Rules, Trends, And Periodic Table Insights
Introduction to Electronegativity
Electronegativity describes the ability of an atom to attract electrons during the formation of a chemical bond. This property plays a critical role in determining whether the bond is ionic or covalent.
Rules Governing Electronegativity:
1.Higher electronegativity in non-metals: Non-metals exhibit a higher electronegativity than metals. For example, fluorine (F) is the most electronegative element, whereas elements such as Caesium (Cs) register very low electronegativity.
(2)The electronegativity increases across a period: When moving from left to right in the periodic table, electronegativity increases. This occurs because of the increased nuclear charge, which attracts the electrons more effectively.
3.Electronegativity decreases down a group: As one proceeds down a column, the electronegativity decreases. This is due to an increase in atomic radius, which places the valence electrons farther from the nucleus, thereby reducing the attractive force.
4. thePauling Scale: Electronegativity is commonly measured using the Pauling Scale. Fluorine, with a value of 3.98, is identified as the element with the highest electronegativity.
Examples of Electronegativity:
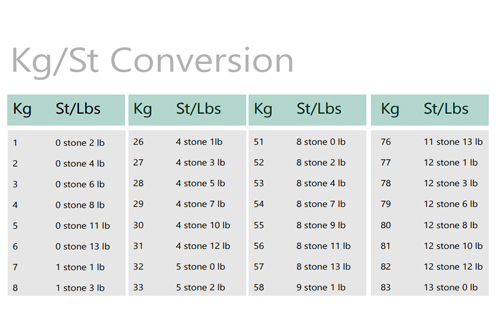
|
Element |
Electronegativity (Pauling Scale) |
|
Fluorine (F) |
3.98 |
|
Oxygen (O) |
3.44 |
|
Nitrogen (N) |
3.04 |
|
Chlorine (Cl) |
3.16 |
|
Carbon (C) |
2.55 |
|
Hydrogen (H) |
2.20 |
|
Sulphur (S) |
2.58 |
|
Caesium (Cs) |
0.93 |
|
Calcium (Ca) |
1.00 |
|
Francium (Fr) |
0.70 |
Periodic Table Insights
lFluorine is the element with the highest electronegativity, owing to its small atomic radius and high nuclear charge.
lCaesium and Francium exhibit the lowest electronegativity and are therefore highly electropositive.
lBond Type Prediction: The difference in electronegativity between two atoms assists in forecasting the bond type:
lIonic Bonds occur when the difference exceeds 1.7.
lCovalent Bonds form when the difference is below 1.7.
Electronegativity is crucial for understanding molecular structure, reactivity and the nature of the bonds formed between atoms. Further information is available at Stanford Advanced Materials (SAM).
Frequently Asked Questions
What is electronegativity?
Electronegativity refers to the ability of an atom to attract electrons within a chemical bond. It influences whether the resultant bonds are ionic or covalent.
What trends are observed in electronegativity across the periodic table?
Electronegativity increases across a period (from left to right) and decreases down a group (from top to bottom). This is attributed to increasing nuclear charge and atomic size.
Which element possesses the highest electronegativity?
Fluorine has the highest electronegativity, with a value of 3.98 on the Pauling Scale.
How does electronegativity affect bond polarity?
The difference in electronegativity between two atoms determines the polarity of a bond. A large difference results in an ionic bond, whereas a small difference gives rise to a polar covalent bond.
Why does electronegativity decrease down a group?
Electronegativity decreases down a group because the atomic radius increases, thereby positioning the valence electrons further from the nucleus and reducing the attractive force on the bonding electrons.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us


 Chin Trento
Chin Trento