Lithium: Element Properties And Uses
Understanding Lithium the Element
What Is Lithium?
Lithium, atomic number 3 and symbol Li, is the lightest metal in the periodic table and an alkali metal. Over the two centuries since its discovery as a laboratory curiosity, lithium has developed into a globally traded industrial commodity. Currently, lithium refining and production—through electrolysis of salt or milling of minerals such as spodumene—form the basis for a multibillion-pound industry in energy storage, space exploration, and electronics.

Chemical Properties Description
Lithium's chemical properties reflect its physical properties in being equally distinctive:
•Reactivity: Lithium vigorously reacts with water to form lithium hydroxide (LiOH) and hydrogen gas. For instance, a piece of small lithium submerged in water can develop visible effervescence within seconds.
•Alkaline Nature: As another alkali metal, lithium readily donates its one outer electron to generate the Li⁺ ion, thereby becoming an important participant in electrochemical reactions, including lithium-ion batteries.
• Flammability: Lithium ignites with a characteristic red flame, which engineers must consider while working with and storing bulk lithium.
Physical Properties Data Table
|
Property |
Value |
|
Atomic Number |
3 |
|
Atomic Weight |
6.94 g/mol |
|
Melting Point |
180.54 °C |
|
Boiling Point |
1590 °C |
|
Density |
0.534 g/cm³ |
|
Appearance |
Silvery-white metal |
|
Hardness |
Soft |
|
Electrical Conductivity |
High |
|
Thermal Conductivity |
High |
Discovery of Lithium
Lithium was first discovered in 1817 in petalite, on the island of Utö, Sweden. Arfvedson's analysis identified a new element distinct from sodium or potassium. Metallic lithium was subsequently separated by electrolysis of lithium salts, permitting further analysis of its physical and chemical properties. Over time, the unique combination of lithium's lightness, high reactivity, and electrochemical potential directed its application from energy storage to industrial greases and medicine.
Alloys and Lithium Compounds
• Lithium-Aluminium Alloys: Extremely light and hard, used in aircraft components and sports car parts.
• Lithium-Copper Alloys: Improve electrical conductivity and are commonly used in electronic connectors and circuits.
• Lithium-Iron Phosphate (LiFePO₄): Frequently found in electric car battery cathodes due to stability and long cycle life.
• Lithium Hydroxide (LiOH) and Carbonate (Li₂CO₃): Vital in rechargeable batteries, lubricating grease, and industrial chemical processes.
• Lithium Chloride (LiCl): Used in desiccants, air conditioning systems, and chemical synthesis.
For example, Tesla and other electric vehicle manufacturers utilise lithium-iron phosphate (LFP) batteries in certain car fleets, where thermal stability and energy density are critical for ensuring safety and functionality.
Common Applications
Various applications of lithium include:
1. Batteries: Lithium-ion and lithium-polymer batteries dominate the market in portable electronics, electric vehicles, and renewable energy storage systems due to their high energy density.
2. Alloys: Lithium lightens aluminium and copper alloys, enhancing aerospace efficiency without compromising strength.
3. Medicinal Uses: Lithium carbonate stabilises patients with bipolar disorder, demonstrating lithium's relevance beyond industrial applications.
4. Heat-Resistant Glass and Ceramics: Lithium enhances thermal shock resistance and strength.
5. Lubricating Greases: Lithium greases perform effectively at high temperatures and pressures, essential in manufacturing and the automotive sector.
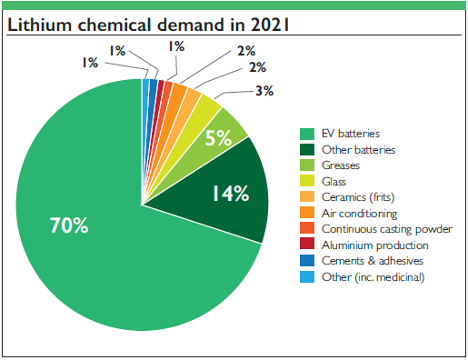 [1]
[1]
Preparation Processes
Lithium is primarily obtained through:
1. Lithium metal recovery from LiCl or LiF via electrolysis to yield high purity for market applications.
2. Mining minerals: Spodumene and petalite are processed to produce lithium carbonate or hydroxide for battery and chemical production.
Frequently Asked Questions
What are the most common applications for lithium?
Primarily in rechargeable batteries, light alloys, lubricating greases, and pharmaceuticals.
How is lithium extracted?
Through electrolysis of lithium salts or from spodumene mineral ores.
Is lithium reactive?
Yes, particularly with water, yielding LiOH and hydrogen gas.
What are lithium-ion batteries?
They provide power to smartphones, laptops, electric vehicles, and renewable energy storage systems due to high energy density and long cycle life.
Reference:
[1] International Lithium Association (2023, October 13). Lithium 101. Retrieved 17/07/2025, from https://lithium.org/lithium-101/

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us



 Chin Trento
Chin Trento



