The Evolution Of Electric Vehicle Batteries: From Lead-Acid To Lithium-Ion
Introduction
The development of electric vehicles (EVs) has progressed significantly over recent years. At the centre of this advancement is the battery technology that powers these vehicles. This article outlines the historical progression of batteries for EVs, from the early lead-acid batteries to modern lithium‑ion technology.
Lead‑Acid Batteries: Initial Models
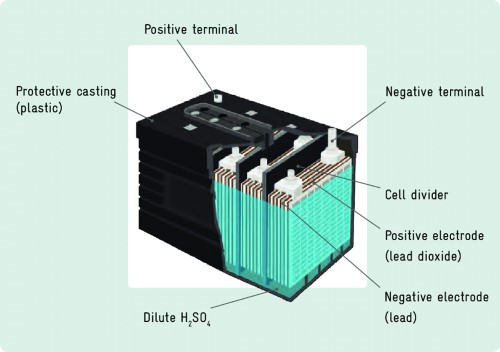
In the late 19th century, lead‑acid batteries emerged as the first types used in electric vehicles. These batteries utilised a chemical reaction between lead dioxide (positive plate), lead sponge (negative plate) and a sulphuric acid electrolyte to generate electrical energy. They were employed in a range of applications.
 [1]
[1]
Figure 1. Typical Construction of Lead‑Acid Batteries
Early electric vehicles were restricted by the technology available at that time. The limited energy density and range meant that they were not suitable for long journeys or interurban travel. In addition, charging infrastructure was not established, and the charging process was time‑consuming. This lack of convenience further restricted the practical use of electric vehicles.
Despite these limitations, lead‑acid batteries continue to be used today. They are frequently applied in various fields, for example as starting batteries for vehicles, in uninterruptible power supplies (UPS) and in off‑grid renewable energy systems.
Nickel‑Metal Hydride Batteries: A Step Forward
In the early 20th century, Thomas Edison developed the nickel‑iron battery. This rechargeable battery is based on an electrochemical reaction between a positive nickel oxyhydroxide electrode (NiOOH), a negative metal hydride electrode (MH) and an alkaline electrolyte. Although nickel‑metal hydride (NiMH) batteries offer a higher energy density and extended range, they did not become the industry standard for electric vehicles.
Lithium‑Ion Batteries: A Turning Point
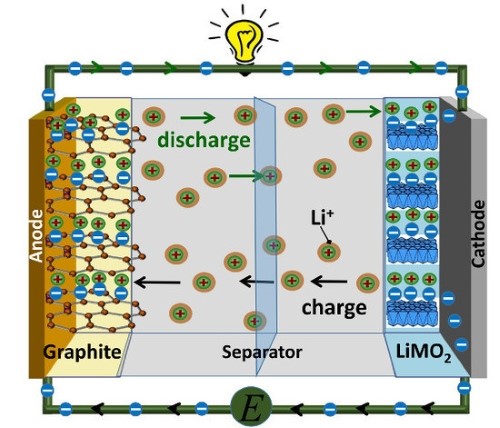
In the 21st century, lithium‑ion batteries were widely adopted, marking an important change in battery technology for electric vehicles. They offer a higher energy density, greater range and faster charging, thereby establishing themselves as the standard for modern EVs. During the charging process, lithium ions (Li+) migrate through the electrolyte from the cathode to the anode, thereby storing energy. During discharge, the Li+ ions return to the cathode, producing an electric current.
 [2]
[2]
Figure 2. Structure of Lithium‑Ion Batteries
Lithium‑ion batteries are characterised by a high energy density, durability and a low self‑discharge rate, allowing them to maintain charge for extended periods. The cathode is manufactured from several materials such as lithium cobalt oxide (LiCoO2) for consumer electronics, lithium iron phosphate (LiFePO4) for electric vehicles and lithium nickel cobalt manganese oxide (NCM) or lithium nickel cobalt aluminium oxide (NCA) for a balanced performance in both energy and power density.
This range of options enables the use of lithium‑ion batteries in numerous applications, from household appliances to electric vehicles, and supports ongoing research. Such research includes the development of solid‑state batteries and efforts to minimise cobalt usage, thereby reducing the environmental and societal impacts of cobalt extraction.
The Future of EV Batteries:
The development of EV batteries is ongoing, with future improvements expected to include higher energy density, faster charging and enhanced sustainability.
lSolid‑State Batteries: The development of solid‑state lithium‑ion batteries represents a notable advancement in battery technology. These batteries offer increased energy density, improved safety and a longer service life compared with conventional liquid electrolyte batteries.
lReduced Cobalt: Given the ongoing environmental and ethical concerns associated with cobalt extraction, efforts are underway to minimise or entirely eliminate cobalt in lithium‑ion batteries. These measures aim to develop more sustainable battery chemistries and reduce the environmental and social impacts linked to cobalt extraction.
lFast Charging: Advances in fast charging technology have reduced charging times significantly, thereby addressing one of the principal obstacles to electric vehicle adoption.
Conclusion
In summary, the progression of battery technology for electric vehicles has been marked by measurable improvements, with lithium‑ion technology currently dominating the market. As technology advances further, future EV batteries are expected to offer higher energy density, faster charging and improved sustainability.
Stanford Advanced Materials (SAM) is a leading supplier within the Li‑Ion Battery Family. Please submit an enquiry if you are interested.
Reference:
[1] Manhart, Andreas & Magalini, Federico & Hinchliffe, Daniel. (2018). End‑of‑Life Management of Batteries in the Off‑Grid Solar Sector. How to deal with hazardous battery waste from solar power projects in developing countries? Publication commissioned by: GIZ Sektorvorhaben Konzepte für nachhaltige Abfallwirtschaft und Kreislaufwirtschaft; produced in collaboration with Energising Development (EnDev).
[2] Madian, M.; Eychmüller, A.; Giebeler, L. Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries. Batteries 2018, 4, 7. https://doi.org/10.3390/batteries4010007

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators




 Chin Trento
Chin Trento

