Calcium Carbonate (CaCO₃) Crystal Substrates: Properties and Applications
It's perhaps most familiar as the main ingredient in limestone, marble, coral skeletons, and pearls. It's been extensively used in industry for applications such as a filler material in paints for its opacity and binding properties and as a filler and white pigment in paper products. Much less familiar—is quickly gaining in significance in high-end research and applications engineering—is single-crystal or oriented-crystal calcium carbonate.

Fig.1 Schematic crystal structure of the CaCO3 polymorphs aragonite and calcite [1]
Crystal Structure and Polymorphism
Calcium carbonate exists as three main crystalline polymorphs. These include calcite, aragonite, and vaterite. These have different lattice symmetries and stabilities. In addition, they have different applicative values as a substrate.
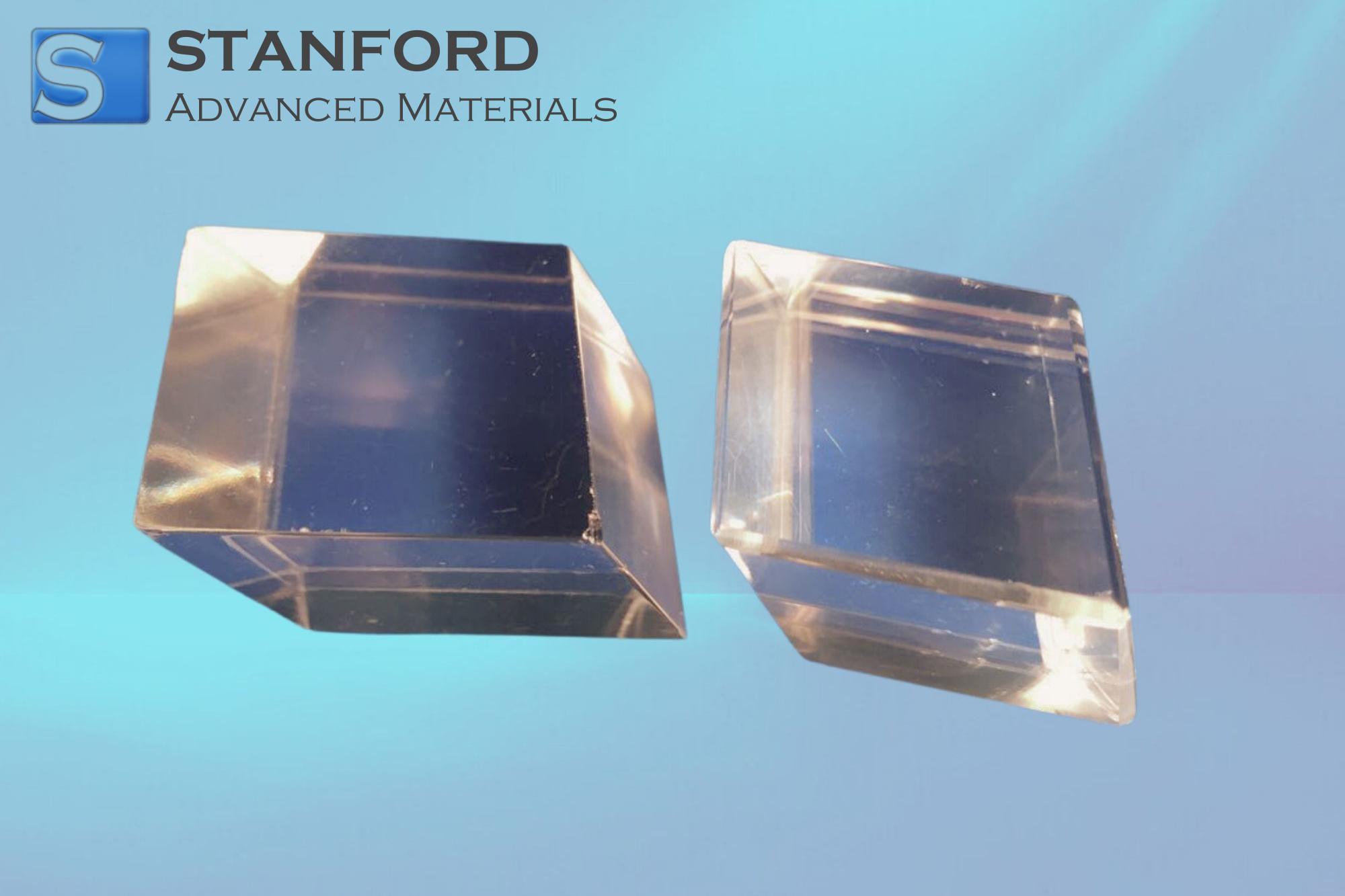
- Calcite has the highest thermodynamical stability at normal conditions, and its crystal structure belongs to the trigonal system. This particular polymorph of calcite is most preferred for crystal substrates, as it is possible to obtain large, optically transparent single crystals, and its surface can be polished to atomic scale flatness. The (104) surface of calcite is a typical model surface in mineral physics.
- Aragonite has an orthorhombic crystal system and is metastable under room-temperature conditions. It has more dense atomic packing and relative hardness compared to calcite. Aragonite is of special importance in the studies involving biomineralisation as it mimics the mineral component in nacre and many biological shells.
- Vaterite is the least stable polymorph, with hexagonal symmetry. It is readily convertible to calcite or aragonite forms and is not commonly used as the bulk crystal substrate material. However, it is intensively used for surface science research as well as for some medical research due to its high surface area and high pore volumes.
Regarding this, calcite single crystal CaCO₃ crystal substrates make up the vast number of crystal substrates being used.
Physical, Optical, and Chemical Properties
The attractiveness of calcium carbonate substrate is attributed by the balance and uniqueness of its properties.
Crystallographically, CaCO₃ single crystals have both long-range order and a fixed lattice direction. Such properties make it possible to produce a substrate with well-aligned crystal planes, which is a crucial aspect associated with epitaxial growth and experiments for surface reconstruction.
Optically, calcite is highly birefringent, and its refractive index difference, Δn, for the visible region is about 0.17. Such an optical characteristic is the basis for its applications in polarisation optics, such as wave plates and beam displacers. For high-purity calcite crystals, they are transparent to the entire visible region and part of the near infrared region.
From a mechanical point of view, calcium carbonate has low hardness, with a Mohs value of around 3. Although this makes it much easier to scratch than quartz or a sapphire, this property makes it very amenable to cutting, lapping, and polishing into thin slices or wafers. Thin substrates with dimensions ranging from a few millimetres down to several hundred micrometres can be prepared.
Chemically, calcium carbonate is stable in neutral to slightly alkaline environments but is reactive in acidic environments, producing CO₂. It also readily reacts with water, ions, and biological molecules on its surfaces, making it an attractive substrate in studies related to adsorption and mineral solution interfaces. Calcium carbonate is also non-toxic and biocompatible.
Growth and Substrate Preparation
The preparation of quality CaCO₃ crystal substrates requires controlled crystal growth and cutting and finishing operations.
Single crystals of calcite and aragonite can be obtained by evaporation or controlled precipitation of aqueous solutions or hydrothermal crystallisation. Parameters such as temperature, pH, supersaturation, and organic additives have distinct effects on crystal growth parameters such as crystal size and polymorphism. To produce research-grade wafers, it is necessary to have crystals of relatively low impurity and twin density.
Once they have been grown, crystals are aligned via X-ray diffraction (XRD) analysis in order to locate particular surfaces of the crystal lattice, like the (104) surface of calcite. Diamond-saw sections are used to section crystals into planes, which are then lapped and polished to produce flatness along with surface roughness on the nanometric scale. The surfaces may be etched or functionalised with organic molecules, polymers, or thin films, depending upon requirements.
Applications in Research & Technology
Surface Science and Mineral Physics
Calcite (104) substrates are one of the most well-studied mineral substrates. They are standard systems for kinetic studies on dissolution and precipitation, ion adsorption, surface reconstruction, and crystal growth. Such studies hold prime importance in understanding geological processes, scaling, and biomineral formation.
Biomineralisation and Biointerfaces
Calcium carbonate substrates are commonly employed for research into protein-, peptide-, and polysaccharide-mediated nucleation as well as growth of minerals within biological systems. Oriented calcite substrates as well as aragonite substrates are useful for model study since these substrates are closely related from a structural viewpoint for shell formation studies, studies involving the interface between bones and minerals, as well as cell adhesion on substrates with minerals.
Optical Components
High-purity calcite crystals are used in polarisation optics such as Nicol prisms, Glan-Taylor prisms, and wave plates. Thin plates of polished calcite are used in integrated optical experiments and in studies of anisotropic light-matter interaction.
Thin Film Growth and Hybrid Interfaces
CaCO₃ substrates with an oriented crystal structure can serve as a template for the formation of organic layers by epitaxial or quasicrystal growth methods, biomolecular layers, and nanostructures of other materials. The use of such templates is of great interest for hybrid organic-inorganic materials and nanostructures.
Microfabrication and Patterning
The medium hardness as well as the ability to react with chemicals makes calcium carbonate susceptible to FIB milling, laser ablation, and wet chemical etching techniques. CaCO₃ patterns are used for the preparation of microfluidic chips, biosensors, as well as for template-assisted nanostructuring.
Environmental and Geochemical Modelling
CaCO₃ substrates have been extensively used as a model of natural surfaces to examine CO₂ sequestration, heavy metal adsorption, ocean acidification, and scale formation processes.
Conclusion
Calcium Carbonate Crystal Substrates have the particular specificity in-between optical crystals and semiconductor wafers. The properties of calcium carbonates like crystallographic ordering, optical anisotropy, high activity at the surface level, along with biocompatibility, make it difficult to imagine surface sciences or biomineral sciences without it. For more optical products, please check Stanford Advanced Materials (SAM).
Reference:
[1] Soldati, Analia & Jacob, Dorrit & Glatzel, Pieter & Swarbrick, Janine & Geck, Jochen. (2016). Element substitution by living organisms: The case of manganese in mollusc shell aragonite. Scientific Reports. 6. 22514. 10.1038/srep22514.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us

 Dr. Samuel R. Matthews
Dr. Samuel R. Matthews
