What Metal Is A Good Conductor Of Heat
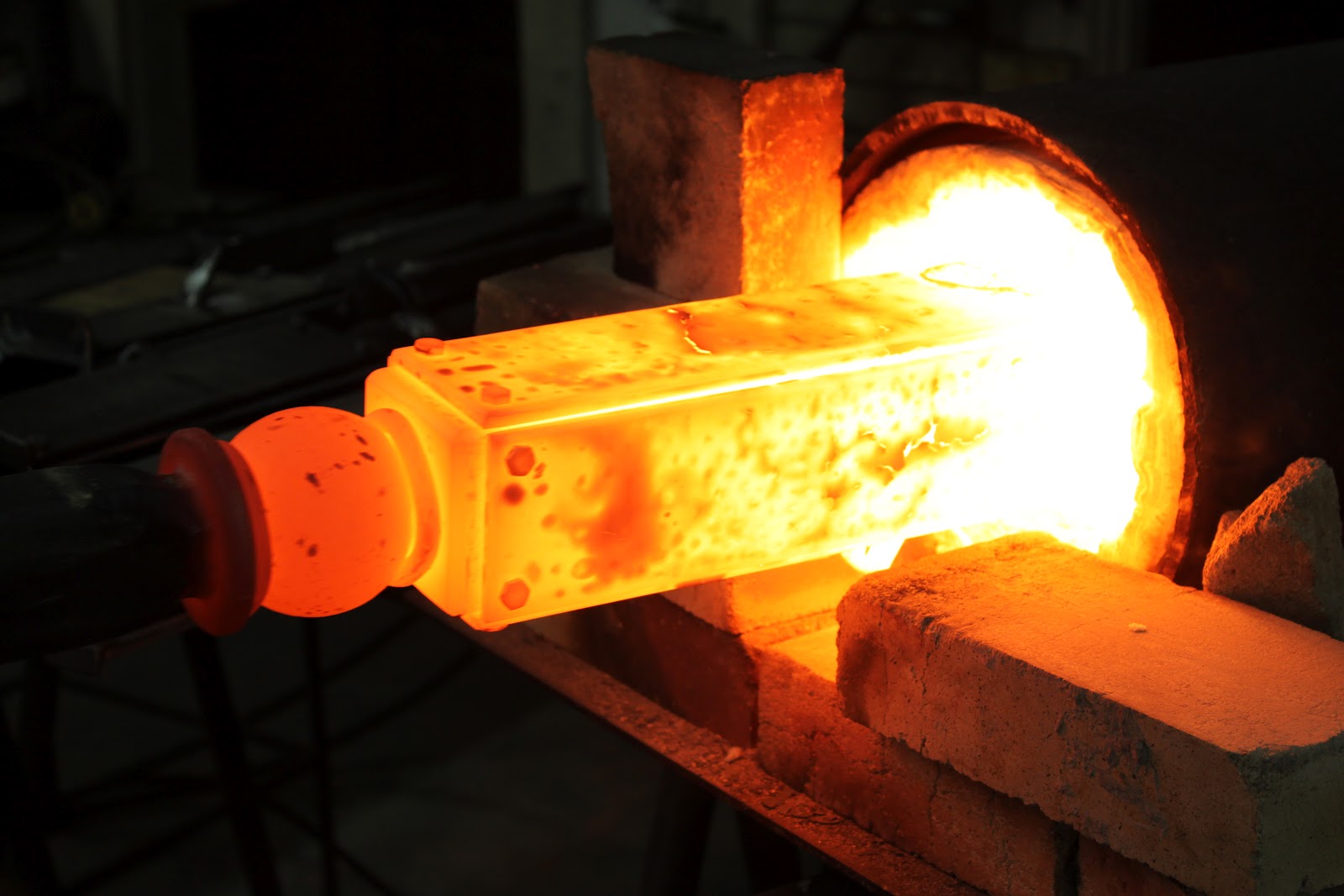
Generally, metals exhibit a shiny appearance and reflect most of the incident light. They are malleable and ductile, meaning they can be deformed under pressure without fracturing. Their melting points span a wide range; for example, mercury is liquid at room temperature, gallium melts in the hand, and tungsten has a melting point of approximately 3 400 °C. Metals also demonstrate high thermal and electrical conductivity compared with non-metal materials such as plastics, ceramics, rocks, and solid salts.

aluminium foil, steel wool, a paper clip, copper, a carbon rod, and a graphite pencil all conducted electricity and exhibited metallic characteristics. In contrast, a glass rod, plastic, rubber, and wood conducted electricity poorly. Most samples with metallic characteristics contained metallic bonds; carbon was an exception. Carbon, which is classified as a non-metal, forms covalent bonds. In this experiment, it demonstrated conduction properties. Graphite is the only non-metallic element known to conduct electricity.
Graphite consists of layers of hexagonal arrangements of carbon atoms that are connected by covalent bonds. Weaker pi bonds exist between these layers, thereby allowing electrons to move freely. This electron movement explains the conduction properties observed in graphite. Copper contains metallic bonds and therefore conducts electricity, whereas rubber comprises covalent bonds and does not conduct electricity, thereby protecting the user from electric shock.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us

 Chin Trento
Chin Trento


