Everything You Need To Know About Titanium Oxides
Introduction
Titanium oxides are compounds that consist of titanium and oxygen. They occur in two primary forms: Titanium Dioxide (TiO₂) and Titanium Monoxide (TiO). This article examines the properties, synthesis methods, applications, and environmental impacts of titanium oxides.
Types of Titanium Oxides
1. Titanium Dioxide (TiO₂)
TiO₂ is a white, odourless powder. It has a high refractive index and strong ultraviolet light absorption. It exhibits photocatalytic activity and chemical stability. It is non‐toxic.
TiO₂ occurs in three main polymorphs: Anatase, Anatase, Rutile and Brookite. Anatase and Rutile are the most common. Rutile is thermodynamically stable; consequently, Anatase converts to Rutile upon heating.

2. Titanium Monoxide (TiO)
TiO has a metallic lustre and conducts electricity. It crystallises in a rock‐salt structure. It is mainly used in specialised applications such as thin films and coatings.
Synthesis of Titanium Oxides
1. Titanium Dioxide (TiO₂)
- Sulphate Process: Ilmenite (FeTiO₃) is reacted with sulphuric acid. Titanium sulphate is produced. Hydrolysis and calcination yield TiO₂.
- In the Chloride Process, ilmenite or rutile is chlorinated at high temperatures. Titanium tetrachloride (TiCl₄) forms. It is then oxidised to produce TiO₂.
- A modified approach is the Sol–gel Process. Titanium alkoxides undergo hydrolysis and polymerisation, followed by drying and calcination. This method yields TiO₂ nanoparticles with controlled size and morphology.
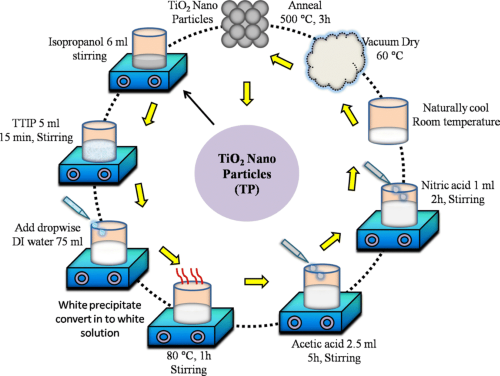 [1]
[1]
2. Titanium Monoxide (TiO)
Reduction methods are employed for the synthesis of TiO. TiO₂ is reduced with hydrogen, or titanium and oxygen are combined under controlled conditions.
Applications of Titanium Oxides
1. Titanium Dioxide (TiO₂)
- Pigments: TiO₂ is used in paints, coatings, plastics, paper, and inks because of its opacity and brightness.
- Sunscreens and Cosmetics: TiO₂ is incorporated into products that protect against ultraviolet radiation.
- Photocatalysis: TiO₂ is utilised in air and water purification, self‐cleaning surfaces and antibacterial coatings.
- Electronics: TiO₂ is used in varistors and capacitors due to its dielectric properties.
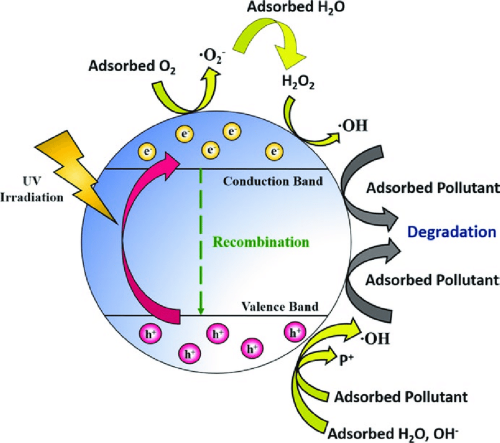 [2]
[2]
2. Titanium Monoxide (TiO)
Thin Films and Coatings: TiO is used to produce thin layers for optical coatings, semiconductors and sensors. Its electrical conductivity and thermal stability are beneficial for these applications.
Environmental Impacts and Safety
Environmental Impacts: Although TiO₂ is generally considered safe for human health and the environment, its extensive use raises concerns regarding nanoparticle pollution. TiO₂ nanoparticles may enter watercourses and affect aquatic life. Regulations and guidelines exist for its production and disposal.
Human Health: TiO₂ is subject to regulatory controls in food, cosmetic and pharmaceutical applications. Inhalation of TiO₂ dust may pose risks to the respiratory system. Appropriate handling and protective measures are mandatory in industrial settings.
Photocatalytic Activity: The photocatalytic properties of TiO₂ generate reactive oxygen species (ROS). ROS degrade pollutants. However, excessive ROS can cause oxidative stress in living organisms.
Future Perspectives and Research
Researchers are developing materials based on TiO₂ with improved characteristics for energy storage, photovoltaics and photocatalysis. Doping TiO₂ with other elements can improve efficiency. New synthesis methods are being explored to control particle size and morphology. Efforts are underway to develop sustainable and environmentally friendly production methods. These include the application of green chemistry principles, recycling of TiO₂ waste and enhancing the efficiency of photocatalytic processes.
Conclusion
Titanium oxides, specifically TiO and TiO₂, play a significant role in various industries. TiO is deployed in specialised applications such as thin films and coatings because of its metallic lustre and electrical conductivity. TiO₂ is widely used in pigments, sunscreens, cosmetics, photocatalysis and electronics due to its high refractive index, strong ultraviolet light absorption, photocatalytic activity and chemical stability. Technological development ensures that titanium oxides remain important in materials science and industrial applications. Stanford Advanced Materials (SAM) supplies titanium products at competitive prices. Their range includes photocatalytic nano titanium dioxide powder, nano TiO₂ powder for lithium batteries, nano TiO₂ powder for ceramics, as well as anatase and rutile forms of titanium dioxide. For further details, please visit their homepage.
|
Aspect |
Titanium Monoxide (TiO) |
|
|
Properties |
White, odourless powder, high refractive index, strong ultraviolet light absorption, photocatalytic activity, chemical stability, non‐toxic. |
Metallic lustre, electrical conductivity, rock‐salt structure. |
|
Synthesis Methods |
Sulphate Process: Reaction of ilmenite (FeTiO₃) with sulphuric acid, followed by hydrolysis and calcination to obtain TiO₂. |
Reduction Methods: Reduction of TiO₂ with hydrogen, or direct combination of titanium and oxygen under controlled conditions. |
|
Applications |
Pigments: Used in paints, coatings, plastics, paper and inks. |
Thin Films and Coatings: Applied in optical coatings, semiconductors and sensors. |
Reference:
[1] Pawar, V., Kumar, M., Dubey, P., Singh, M. K., Sinha, A. & Singh, P. (2019). Impact of synthesis route on the structural, optical and electrical properties of TiO₂. Applied Physics A. 125. 10.1007/s00339-019-2948-3.
[2] Leong, K., Ching, S., Pichiah, S. & Ibrahim, S. (2016). Light Driven Nanomaterials for Removal of Agricultural Toxins.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Chin Trento
Chin Trento


