Everything You Should Know About MoO3 Nanoparticles
Introduction
Molybdenum trioxide (MoO3) is one of the transition metal oxides with the chemical formula MoO3(H2O)n (n = 0 to 3). MoO3 is employed in a variety of applications, for example as a photocatalyst, in optics, in gas detection, in batteries and in electronic devices. Anhydrous MoO3 forms a distorted “MoO6” octahedral structure, and its orthorhombic crystal is presented in Figure 1. The green spheres denote molybdenum and the red spheres denote oxygen. MoO3 exists in three distinct crystal phases: α‐orthorhombic, β‐monoclinic and h‐hexagonal. The different MoO3 structures result in varying physical and chemical properties. The h‐MoO3 retains phase stability until 436 °C, whereas the α‐MoO3 undergoes an irreversible phase transition below 436 °C [1].

Figure 1: Structure of MoO6 octahedra
Synthesis of MoO3 Nanostructures and Discussion on Synthesis by Solution Combustion
Several synthetic methods are available for the production of molybdenum trioxide nanoparticles:
Hydrothermal synthesis: Molybdenum salts, such as ammonium molybdate, react with hydrogen peroxide in an aqueous solution at elevated temperature and pressure to yield MoO3 nanoparticles.
Solvothermal synthesis: Molybdenum salts react with an organic solvent such as ethanol under high temperature to form MoO3 nanoparticles.
Co-precipitation: A molybdenum salt solution reacts with a precipitating agent, for example a metal hydroxide or carbonate, at a set pH, resulting in the precipitation of MoO3 nanoparticles from the solution.
Solution combustion synthesis: A mixture of a molybdenum salt and an organic fuel-oxidiser formulation is combusted at high temperature to produce MoO3 nanoparticles.
Additional synthesis routes exist but are not covered here. For further information or enquiries, please contact Stanford Advanced Materials. Even within one synthesis method, varying parameters yield different forms of MoO3 nanostructures. One example is the solution combustion synthesis.
Dissolve ammonium heptamolybdate (NH4)6Mo7O24·4H2O in distilled water. Mix this solution with an organic reagent. In these experiments, urea, EDTA, PEG 200 and sorbitol are employed as separate organic additives. Heat and stir the solution until precipitates form. The final step involves heating the precipitates to remove organic additives and other impurities [2].
Ammonium heptamolybdate (AHM) is a large, complex molecule frequently used as a precursor in the production of molybdenum compounds. The chemical equation for the combustion synthesis of the AHM solution is shown below.
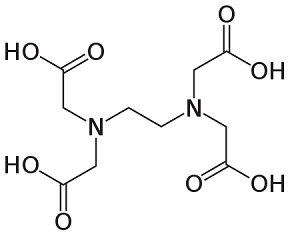
MoO3 can form without additives; however, the additives crucially influence the crystal growth and the MoO3 nuclei. Examination by scanning electron microscopy (SEM) reveals variations in the microstructures produced by the different organic additives. Urea produces a more spherical morphology than the other three additives. PEG 200 results in larger, submicrometre particles that are less spherical. Sorbitol and EDTA yield distinctly rod-like nanostructures [2]. This outcome is quantified by the measurements obtained in the study. Figure 2 presents the chemical structures of the four organic additives used in the experiments. Urea contains nitrogen with a non-bonding electron pair. Both PEG 200 and sorbitol contain oxygen bound to hydroxyl groups. EDTA includes two nitrogen atoms with non-bonding electron pairs and oxygen from hydroxyl groups. In the formation of ligands, the nitrogen atoms, each with two free electrons, exert a stronger attraction on molybdenum than the oxygen atoms. Consequently, urea is capable of attracting Mo from AHM to form nuclei of smaller size compared to PEG 200 and sorbitol [2].
EDTA comprises two nitrogen atoms with non-bonding electron pairs and four oxygen atoms in hydroxyl groups. Although EDTA might initially appear well suited as an additive for the production of MoO3 nanoparticles, its large and complex molecular structure introduces significant steric hindrance. This steric effect inhibits the ability of EDTA’s nitrogen to attract Mo effectively. Thus, only the oxygen groups participate in the ligand formation, influencing the resulting microstructure of MoO3 [2].
PEG 200 has one oxygen group at each end. Its ability to coordinate with Mo concurrently on both sides is reduced compared to urea. Nevertheless, PEG 200 has a simple molecular structure with minimal steric hindrance. Therefore, forming a ligand is more straightforward with PEG 200 than with EDTA [2].
When one oxygen group of sorbitol binds to Mo, its linear molecular structure prevents simultaneous binding by another oxygen group to a separate Mo atom. Overall, sorbitol proves to be an ineffective additive for the synthesis of MoO3 nanoparticles [2]. Other factors, such as pH, reaction temperature, Mo concentration and the ratio of Mo to additive, also influence the properties of the resulting MoO3 nanoparticles.
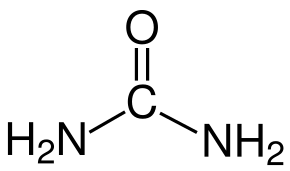
Urea EDTA
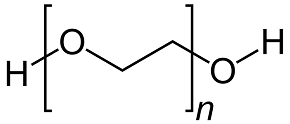
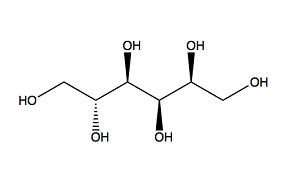
PEG200 Sorbitol
Figure 2: Molecular structures of the organic additives used in the experiments
Applications of MoO3
MoO3 membranes exhibit commendable electrochromic behaviour. When compared with materials such as WO3 and TiO2, MoO3 shows a reduced reaction time. Furthermore, MoO3 assumes a grey colour upon exposure to an electrical stimulus. Its absorption curve is uniform throughout the visible spectrum. The absorption peak occurs at approximately 550 nm, which corresponds closely with the sensitivity range of the human eye. The synthesis of high-quality MoO3 using MoO3 nanoparticles is a subject of considerable study.
PVC is a widely used thermoplastic polymer. However, when combusted it emits dense smoke. The inclusion of transition metal compounds significantly reduces the smoke density. In formulations containing two or more transition metal compounds, the dense smoke typically generated by PVC is substantially reduced. PVC also poses a fire hazard due to the use of plasticisers. MoO3 exhibits effective flame retardant properties. When MoO3 is combined with Cu2O, the overall additive cost is diminished while maintaining the desired properties in cable applications.
MoO3 is an efficient photocatalyst. Unlike conventional methods for treating dye-containing wastewater, photocatalysts based on nanoparticles convert pollutants into harmless products, such as CO2, at measurable rates [3]. The increased surface area provided by the nanoparticles contributes to an accelerated degradation rate.
MoO3 is an n-type semiconductor applicable in gas detection. Metal oxide gas sensors convert gas molecules into an electrical signal, thereby offering a simpler measurement method. In contrast to other metal oxide gas sensors, MoO3 possesses a wide band gap and contains active sites on its surface that react selectively with target gases. MoO3 demonstrates high gas sensitivity. It responds to NH3, H2, CO and other gases at approximately 450 °C. Pure MoO3 membranes do not perform adequately due to their high temperature sensitivity and selectivity. Consequently, by combining MoO3 with other materials, enhanced gas sensitivity may be achieved. For instance, combining MoO3 with V2O5 in membrane production yields high sensitivity at lower operating temperatures (approximately 150 °C) for NO2, NH3, CO, CH4, SO2 and H2.
Numerous applications for MoO3 nanoparticles exist which are not detailed here. Stanford Advanced Materials (SAM) supplies various types of MoO3. For further information regarding MoO3 applications, please contact our technical staff with your application details.
Reference
- Wongkrua, P., Thongtem, T., & Thongtem, S. (2013). Synthesis of h‐ and α‐MoO3 by refluxing and calcination combination: Phase and morphology transformation, photocatalysis and photosensitisation. Journal of Nanomaterials, 2013, Article ID 702679, 8 pages, published 2013. https://doi.org/10.1155/2013/702679
- Parviz, D., Kaz→emeini, M., Rashidi, A. M., & Jafari Jozani, K. (2009). Synthesis and characterisation of MoO3 nanostructures by solution combustion using morphology and size control. Journal of Nanoparticle Research, 12(4), 1509–1521. https://doi.org/10.1007/s11051-009-9727-6
- Thekkethil, A. J., Sreekuttan, S., & Madhavan, A. A. (2021). Application of nano-molybdenum trioxide in thermal energy storage and photocatalysis. Journal of Physics: Conference Series, 2070(1), 012120. https://doi.org/10.1088/1742-6596/2070/1/012120

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators





 Chin Trento
Chin Trento


