PH Scale: Acids, Bases, And Common Materials
pH Scale of Common Acids
|
Acid |
Designation |
10 mM |
|
H2CO3 |
Carbonic Acid |
4.18 |
|
H2CrO4 |
Chromic Acid |
2.33 |
|
H2MoO4 |
Molybdic Acid |
2.94 |
|
H2S |
Hydrogen Sulphide |
4.47 |
|
H2Se |
Hydrogen Selenide |
2.93 |
|
H2SeO3 |
Selenious Acid |
2.47 |
|
H2SeO4 |
Selenic Acid |
1.83 |
|
H2SO4 |
Sulphuric Acid |
1.87 |
|
H3AsO3 |
Arsenious Acid |
5.58 |
|
H3AsO4 |
Arsenic Acid |
2.31 |
|
H3BO3 |
Boric Acid |
5.62 |
|
H3PO4 |
Orthophosphoric Acid |
2.26 |
|
H4SiO4 |
Silicic Acid |
5.91 |
|
HBr |
Hydrobromic Acid |
2.04 |
|
HCl |
Hydrochloric Acid |
2.04 |
|
HF |
Hydrofluoric Acid |
2.65 |
|
HI |
Hydroiodic Acid |
2.04 |
|
HNO2 |
Nitrous Acid |
2.67 |
|
HNO3 |
Nitric Acid |
2.04 |
pH Scale of Common Bases
|
Base |
Designation |
10 mM |
|
Ba(OH)2 |
Barium Hydroxide |
12.22 |
|
Be(OH)2 |
Beryllium Hydroxide |
7.90 |
|
Ca(OH)2 |
Calcium Hydroxide (Lime, CaO·H2O) |
12.20 |
|
CaCO3 |
Calcium Carbonate (Calcite) |
9.91 |
|
Co(OH)2 |
Cobalt(II) Hydroxide |
9.15 |
|
Cr(OH)3 |
Chromium(III) Hydroxide |
7.04 |
|
Cu(OH)2 |
Copper(II) Hydroxide |
7.69 |
|
Fe(OH)2 |
Iron(II) Hydroxide |
9.45 |
|
K2CO3 |
Potassium Carbonate |
11.00 |
|
KHCO3 |
Potassium Hydrogen Carbonate |
8.25 |
|
KOH |
Potassium Hydroxide |
11.95 |
|
Mg(OH)2 |
Magnesium Hydroxide (MgO·H2O) |
10.40 |
|
Na2B4O7 |
Sodium Borate (Borax) |
9.17 |
|
Na2CO3 |
Sodium Carbonate (Soda) |
10.97 |
|
Na2SiO3 |
Sodium Metasilicate |
11.91 |
|
Na3PO4 |
Trinatrium Phosphate |
11.71 |
|
NaHCO3 |
Sodium Hydrogen Carbonate |
8.22 |
|
NaOH |
Sodium Hydroxide |
11.95 |
|
NH4OH |
Ammonium Hydroxide (NH3·H2O) |
10.61 |
|
Ni(OH)2 |
Nickel(II) Hydroxide |
8.37 |
|
Zn(OH)2 |
Zinc Hydroxide |
8.88 |
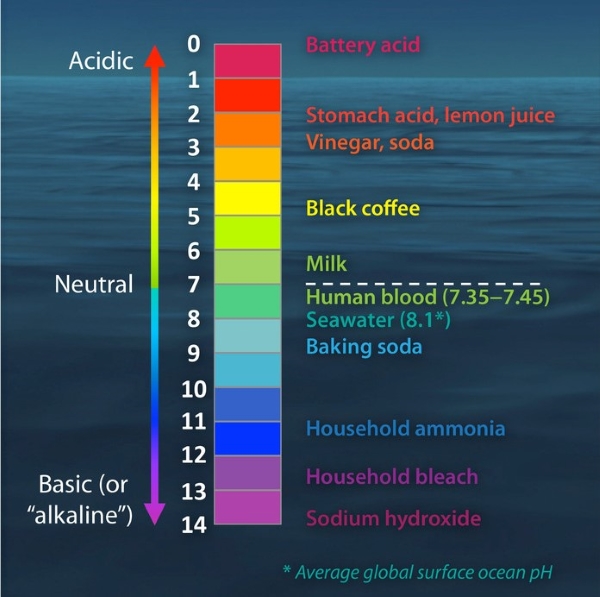
pH Scale of Common Materials
|
pH Value |
Example |
|
0 |
Sulphuric Acid |
|
1 |
Gastric Acid |
|
2 |
Lemon Juice, Vinegar |
|
3 |
Orange Juice, Carbonated Beverages |
|
4 |
Tomatoes, Acidic Rain |
|
5 |
Black Coffee, Bananas |
|
6 |
Urine, Milk |
|
7 |
Distilled Water |
|
8 |
Sea Water, Eggs |
|
9 |
Sodium Bicarbonate |
|
10 |
Large Salt Lake, Milk of Magnesia |
|
11 |
Household Ammonia Solution |
|
12 |
Soap Solution |
|
13 |
Household Bleach, Oven Cleaner |
|
14 |
Liquid Drain Cleaner |
pH Scale: FAQs
1. What is the pH scale?
The pH scale is a measurement system that spans from 0 to 14. It quantifies the concentration of hydrogen ions in a solution. A value of 7 is neutral. Values below 7 are acidic and those above 7 are alkaline.
2. How is the pH value measured?
The pH value is determined using a pH meter or pH paper. The meter employs a glass electrode to quantify hydrogen ion concentration. pH paper contains indicators that change colour when they contact a substance with a specific acid or base concentration.
3. What are acids and bases?
Acids are substances that release hydrogen ions (H⁺) when dissolved in water. Bases are substances that release hydroxide ions (OH⁻) or accept hydrogen ions. This process reduces the hydrogen ion concentration.
4. What are some examples of acids and bases?
Common acids include hydrochloric acid (present in the stomach), citric acid (in citrus fruits) and acetic acid (in vinegar). Common bases include sodium hydroxide, sodium hydrogen carbonate and ammonia.
5. Why is the pH value important?
The pH value is important in chemistry, biology and environmental science. In the human body, specific pH levels are required for enzyme function and homeostasis. In agriculture, pH influences soil quality and plant growth. In industry, such as water treatment, pH regulation is essential for safety and efficiency.
6. How does the pH value affect daily life?
The pH value affects the taste of foods and beverages. Acidic foods, such as lemons, taste sour. Basic substances may have a bitter or soapy taste. It also influences the effectiveness of cleaning agents and the condition of aquatic ecosystems.
7. Can the pH value change?
Yes, the pH value can change due to various factors. The addition of acids or bases, chemical reactions, biological processes and environmental factors may alter the pH of water, soil or even the human body.
Reference:
[1] NOAA Pacific Marine Environmental Laboratory CO2 Program (2021). Die pH-Skala mit einigen gängigen Beispielen [Photo]. https://www.pmel.noaa.gov/co2/file/The+pH+Skala+mit+einigen+üblichen+Beispielen

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators




 Chin Trento
Chin Trento



