Precious Metal Crucible: Types And Applications
Introduction
Precious metal crucibles are essential instruments for various high-temperature applications due to their high resistance to heat and chemical corrosion. Below is an overview of the types and applications of precious metal crucibles:

Types of Precious Metal Crucibles
1. Platinum Crucibles
Platinum crucibles are known for their melting point of 1 768 °C, which makes them suitable for high-temperature applications. They exhibit resistance to oxidation and corrosion and do not react with most substances.
These properties render platinum crucibles indispensable for chemical analysis, glass production, semiconductor manufacturing and the synthesis of high-purity metals.
Further reading: Precious vs. Refractory: An Exploration of Metal Crucibles
2. Gold Crucibles
Gold crucibles have a melting point of 1 064 °C and offer excellent resistance to corrosion as well as good thermal conductivity. They do not react with most chemicals, thereby making them suitable for processes that require low to moderate temperatures, such as fluoride production and melting operations.
1. Silver Crucibles
Silver crucibles, which melt at 961.8 °C, are appreciated for their good thermal conductivity and chemical resistance, particularly against fluorine and its compounds. They are used in the preparation of samples for analyses involving fluorine compounds and in specific chemical reactions where a moderate melting point is beneficial.
2. Palladium Crucibles
Palladium crucibles have a melting point of 1 554.9 °C and provide strong resistance to oxidation and corrosion. They are chemically inert and are therefore employed in specialised chemical processes, catalytic research and certain high-temperature melting procedures.
3. Rhodium Crucibles
Rhodium crucibles possess a high melting point of 1 964 °C and offer excellent resistance to both oxidation and corrosion. They remain stable at elevated temperatures and are frequently used in conjunction with platinum for high-temperature applications in glass production and in certain chemical syntheses.
4. Iridium Crucibles
Iridium crucibles have an extremely high melting point of 2 446 °C and are known for their excellent resistance to corrosion and oxidation. They are hard and brittle and are used for extreme high-temperature applications and for specialised high-purity chemical processes.
Applications of Precious Metal Crucibles
-Analytical Chemistry
Precious metal crucibles are essential tools in laboratories for precise chemical analyses, especially in processes that require high temperatures and resistance to contamination. Below are several common applications of precious metal crucibles in analytical chemistry:
- Precious metal crucibles are used to determine the mass of a substance. They withstand the high temperatures required to drive off volatile components, thereby leaving a residue suitable for accurate weighing.
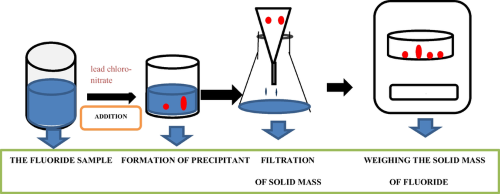 [1]
[1]
2. Melting Procedure
- During sample preparation, melting using fluxes (e.g. lithium borate) requires crucibles that can endure high temperatures and corrosive conditions. Platinum crucibles are commonly employed for melting geological and mineral samples.
3. Ashing
- Ashing involves decomposing organic material at high temperatures to analyse the residual inorganic content. Precious metal crucibles ensure that the residue remains unreacted and its composition is preserved.
4. Chemical Digestion
- For decomposing complex matrices into simpler components, chemical digestion is performed in precious metal crucibles that resist strong acids and oxidising agents, thereby facilitating subsequent analysis.
5. Thermal Analysis
- Techniques such as thermogravimetric analysis (TGA) and differential thermal analysis (DTA) require crucibles that can endure rapid heating and cooling cycles without affecting the sample or the measurement.
6. Preparation and Testing of Catalysts
- Precious metal crucibles are used for the preparation and testing of catalysts, particularly in high-temperature reaction studies. Their chemical inertness ensures that they do not interfere with the measured catalytic properties.
-Glass Production
In glass production, precious metal crucibles are utilised to manufacture high-purity glass and optical fibres where resistance to high temperatures and chemical corrosion is critical. Their specific properties ensure that the glass composition is maintained during various stages of production.
1. Melting and Refining
- Precious metal crucibles are used for melting raw materials and refining the glass by removing impurities. Their high melting point and chemical inertness ensure that the crucible material does not contaminate the glass and that the melt’s composition is preserved.
2. Production of Special Glass
- The manufacture of special glasses such as borosilicate, lead glass and high-purity quartz glass requires the use of platinum or platinum alloy crucibles. These processes require precise control of the melting conditions to achieve specific optical and physical properties.
3. Production of Optical Fibres
- For the production of optical fibres, glass purity is of utmost importance. Platinum crucibles are used to melt preform materials as they introduce no impurities and withstand the high temperatures necessary for fibre drawing.
-Semiconductor Industry
Precious metal crucibles are also employed in the semiconductor industry, particularly in processes that demand a highly pure environment and the growth of single crystals. They are essential for maintaining the integrity and purity of semiconductor materials.
1. Crystal Growth
- Platinum and platinum alloy crucibles are required in the Czochralski process for growing single crystals from silicon, germanium and other semiconductor materials. The high melting point and chemical inertness of platinum prevent contamination during crystal growth, thereby ensuring high-purity crystals.
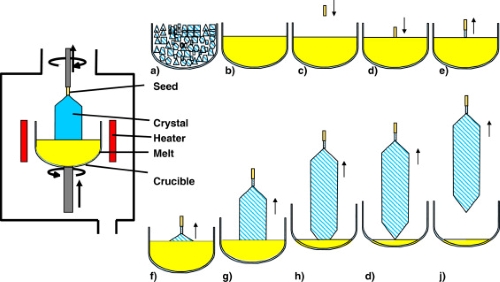 [2]
[2]
2. Doping Procedures
- During semiconductor doping, wherein impurities are intentionally introduced to modify the electrical properties, precious metal crucibles are employed to melt and mix the materials without introducing additional contaminants.
3. Epitaxial Growth
- In epitaxial layer deposition, precious metal crucibles ensure the purity of the starting materials and the quality of the deposited layers.
4. Oxidation and Annealing
- High-temperature processes such as oxidation and annealing, which modify the properties of semiconductor wafers, benefit from the use of platinum crucibles because they can withstand elevated temperatures and corrosive environments.
-Other General Applications
Beyond the semiconductor industry, precious metal crucibles are employed in various other high-temperature and high-purity processes.
1. Synthesis of High-Purity Metals
- Precious metal crucibles are used in the production and refinement of high-purity metals, as they prevent contamination during high-temperature processes.
2. Catalytic Research
- These crucibles are utilised in the investigation and development of catalysts, especially in processes that require high temperatures and exposure to corrosive conditions.
3. Thermal Analysis
- Precious metal crucibles are employed in differential thermal analysis (DTA) and thermogravimetric analysis (TGA) to study materials under controlled temperature conditions.
Conclusion
In summary, precious metal crucibles are indispensable in analytical chemistry, glass production, semiconductor processing and the synthesis of high-purity metals because of their high melting points, chemical inertness and resistance to corrosion. The choice of crucible is determined by the specific requirements of each process. Additional precious metal products and quality crucibles are available at Stanford Advanced Materials (SAM).
Reference:
[1] Khatkar, Rahul & Nagpal, Suman. (2023). Konventionelle und fortschrittliche Nachweismethoden für Fluorid in Wasser: ein Überblick. Umweltüberwachung und -bewertung. 195. 10.1007/s10661-022-10888-x.
[2] J. Friedrich, Methods for Bulk Growth of Inorganic Crystals: Crystal Growth, Reference Module in Materials Science and Materials Engineering, Elsevier, 2016, https://www.sciencedirect.com/science/article/pii/B9780128035818010109

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Chin Trento
Chin Trento


