Basic Types Of Crystal Structure
Description
Understanding the arrangement of atoms in a crystal is fundamental to the study of materials science. Crystal structures determine many of the physical properties of materials, including their strength, conductivity and optical behaviour. This blog post examines various types of crystal structures and the concept of the lattice.
Types of Crystal Structures
Crystals can be categorised according to their atomic arrangement. Common classifications include:
Cubic Crystal System
The cubic system is one of the simplest and most symmetric crystal structures. It has three identical axes that intersect at right angles.
Tetragonal Crystal System
In the tetragonal system, two axes are of equal length while the third axis differs, being either longer or shorter. All axes intersect at 90°.
Orthorhombic Crystal System
The orthorhombic system consists of three axes of differing lengths, all of which intersect at right angles.
Hexagonal Crystal System
Hexagonal crystals have four axes: three lie in the same plane at 120° to one another, with the fourth axis perpendicular to this plane.
Trigonal Crystal System
Similar to the hexagonal system, the trigonal system features three axes in one plane, though it differs in terms of symmetry and atomic arrangement.
The Lattice in Crystal Structures
A lattice is a repeating three-dimensional array of points in space that represents the positions of the atoms in a crystal. The lattice forms the framework upon which the crystal structure is constructed. Understanding the lattice is crucial for determining the material’s properties.
Unit Cell
The smallest repeating unit of a lattice is known as the unit cell. It defines the symmetry and structure of the entire crystal.
Bravais Lattices
There are 14 distinct Bravais lattices, each representing a unique combination of lattice parameters and symmetries in three-dimensional space.
Coordination Number
The coordination number refers to the number of nearest neighbour atoms surrounding a central atom in the lattice. This number affects the stability and bonding characteristics of the crystal.
Comparison of Crystal Structures
|
Crystal System |
Number of Axes |
Axes Lengths |
Angles between Axes |
|
Cubic |
3 |
Equal |
90° |
|
Tetragonal |
3 |
Two equal, one different |
90° |
|
Orthorhombic |
3 |
All different |
90° |
|
Hexagonal |
4 |
Three equal, one different |
120° in the plane, 90° vertical |
|
Trigonal |
3 |
All equal or different |
120° in the plane, 90° perpendicular |
Frequently Asked Questions
What determines the type of crystal structure in a material?
The type of crystal structure is determined by the size, charge and bonding preferences of the atoms or ions that constitute the crystal. These factors dictate the atomic arrangement that minimises the total energy.
How does the lattice affect the properties of a material?
The lattice defines the symmetry and spacing of atoms within the crystal, thereby influencing properties such as electrical conductivity, hardness and optical behaviour.
Can a single element form different crystal structures?
Yes, many elements can crystallise in various structures under different temperature and pressure conditions. For example, carbon crystallises as both diamond and graphite, which exhibit distinct crystal structures and properties.
Why are diagrams significant in the study of crystal structures?
Diagrams provide a visual representation of the atomic arrangement. This facilitates the examination of the symmetry, bonding and overall geometry of the crystal.
What is the significance of the unit cell in a crystal lattice?
The unit cell is the fundamental building block of a crystal lattice. By repeating the unit cell in three dimensions, the entire crystal structure is formed. Understanding the unit cell assists in predicting the material’s properties and behaviour.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
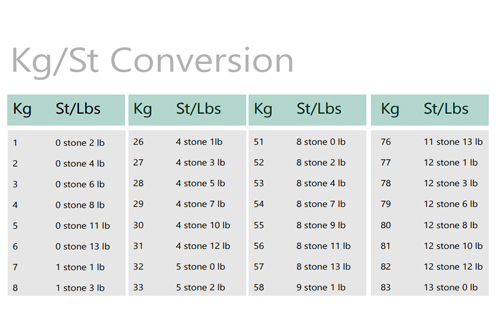
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us


 Chin Trento
Chin Trento