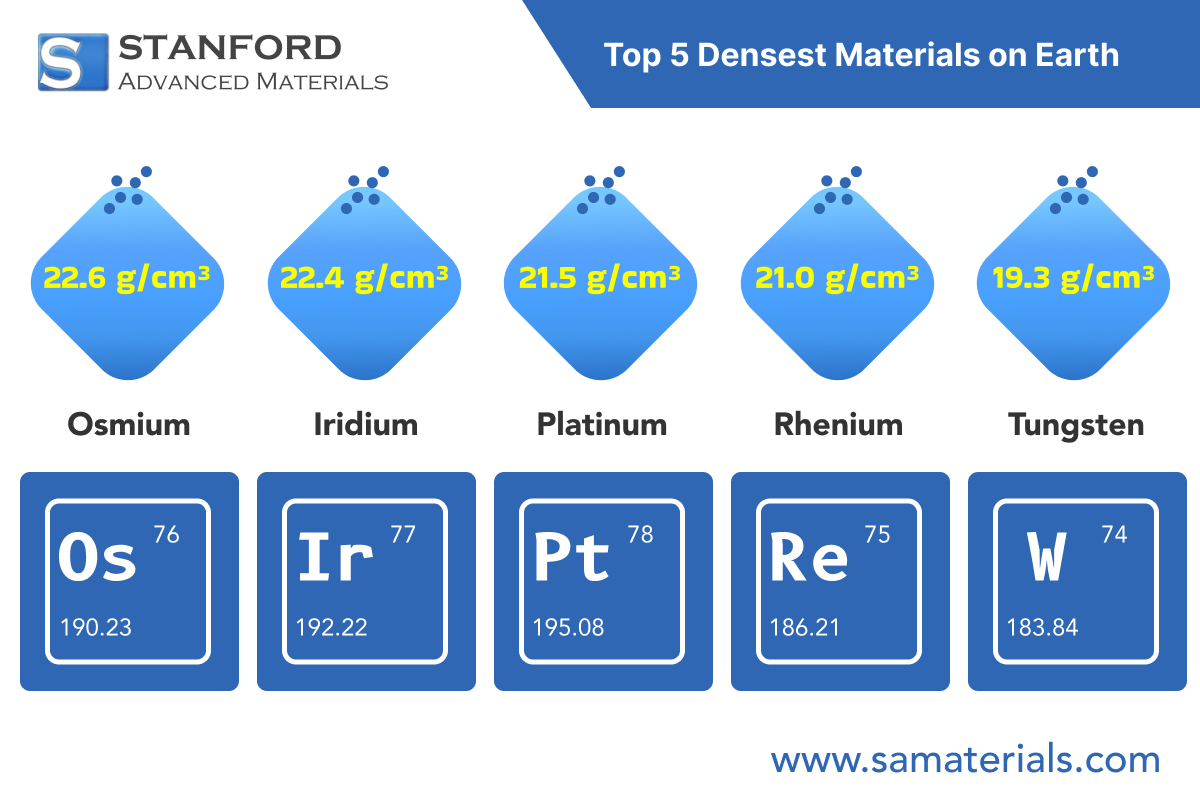
Top 5 Densest Materials on Earth
Introduction
Density is significant in engineering and science. It denotes how closely packed a material is. The denser a material is, the greater its mass per unit volume. Physicists, chemists, and engineers study density to select the appropriate material for specific applications. In everyday life, density correlates with strength, weight, and stability.
Osmium - Approximately 22.6 g/cm³
Osmium is generally regarded as the densest naturally occurring element. It has a density of about 22.6 grams per cubic centimetre. Osmium is characterised by its hardness and blue hue. Osmium is employed when a high level of durability is required, such as in fountain pen tips and electrical contacts. Some fountain pen tips incorporate osmium alloys due to their need to withstand years of use. In scientific apparatus, its durability prevents deformation under extensive use.
Osmium is also utilised in scientific instruments where mass with minimal weight is crucial. Its density provides stability and precision. Osmium is distinctive among metals. Being very dense, even a thin layer can serve as a protective coating for components that experience continuous friction or heat.
Iridium - Approximately 22.4 g/cm³
Iridium possesses a density similar to osmium, approximately 22.4 grams per cubic centimetre. The dense metal demonstrates strong corrosion resistance. Certain high-end electrical contacts and high-performance spark plugs utilise iridium due to its resistance to high temperatures. High durability is greatly preferred in these applications.
Additionally, iridium is applied in high-temperature-resistant laboratory crucibles. Various industrial uses take advantage of its strength and stable nature. Iridium's wear resistance makes it a preferred choice for applications where reliability is critical. Decades of industrial experience have demonstrated that small quantities of iridium significantly enhance equipment performance.
Platinum - The density is approximately 21.5 g/cm³
The density of platinum is approximately 21.5 grams per cubic centimetre. Platinum is also renowned for its aesthetic appeal and resistance to tarnish, rendering it extremely popular in jewellery and investment products. People frequently select platinum for engagement rings and high-end watches. Its appearance is coupled with functional reliability.
In contemporary industry, platinum serves a vital chemical function. It is employed in catalytic converters as a catalyst. The converters eliminate harmful gases from vehicle exhausts. In numerous chemical reactions, a minute quantity of platinum accelerates the reaction without being consumed. Its application in these processes is supported by decades of industrial use. Platinum's reliability in both decorative and industrial contexts has been confirmed over the years.
Rhenium - Approximately 21.0 g/cm³
Rhenium is not as widely recognised as the metals mentioned above but is highly valued in certain industries. It possesses a density of approximately 21.0 grams per cubic centimetre. Rhenium is extensively used in high-temperature superalloys. These alloys find application in jet engine and industrial gas turbine components. The capability of rhenium-containing alloys to perform under demanding conditions ensures safety and efficiency in high-performance equipment.
Moreover, rhenium extends the lifespan of engine components. Its stability contributes to reduced maintenance costs for large machinery. Despite being expensive and rare, rhenium's value in high-demand applications justifies the cost. It exemplifies a material where weight, strength, and heat resistance are effectively balanced.
Gold or Tungsten - Approximately 19.3 g/cm³
Gold and tungsten both have comparable densities, approximately 19.3 grams per cubic centimetre. Gold is recognised for its visual appeal and has historically served as a form of currency. Gold is malleable and soft. Humans have utilised gold for centuries in jewellery, coins, and sculpture. Its density and resistance to corrosion are among its attractions.
Tungsten, in contrast, is a very hard metal. It is used in the manufacture of high-temperature light bulb filaments and robust tools. Tungsten's high melting point, coupled with its density, makes it suitable for applications where durable, hard materials are essential. The choice between tungsten and gold depends on whether strength or aesthetic appeal is of greater significance.
Conclusion
Understanding the densest substances globally provides insights into the domain of high-performance metals. Osmium, iridium, platinum, rhenium, and gold or tungsten all find various applications in daily life and high-tech industries due to their value. Their weight and density render them suitable for specific functions, such as components subject to high wear, stable catalysts in chemical reactions, and highly sought-after decorative items.
Commonly Asked Questions
Q1: What is the densest substance on Earth?
A1: Osmium is the densest natural element, with a density of approximately 22.6 g/cm³.
Q2: How does iridium perform at high temperatures?
A2: Iridium demonstrates resistance to corrosion and high temperatures, making it highly suitable for crucibles and spark plugs.
Q3: What are the general uses of platinum and gold?
A3: Platinum is utilised in catalytic converters and jewellery, while gold is extensively employed in ornaments and coins.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us





 Chin Trento
Chin Trento



