The List Of Support Materials For Palladium Catalysts
Palladium catalysers are essential for many chemical reactions, particularly hydrogenation, oxidation and the formation of carbon–carbon bonds. The performance of palladium catalysers is largely influenced by the choice of support material. The support provides a large surface area for palladium dispersion and affects the stability, activity and selectivity of the catalyst.
Below is a list of the principal support materials used in palladium catalysis. Each support offers specific advantages for various applications:
 [1]
[1]
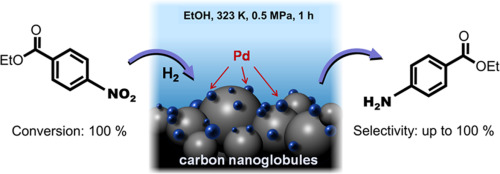
Fig. 1 Palladium catalysers on a carbon support
1. Carbon-based Supports
- Activated Carbon (Pd/C): As one of the most commonly used supports, activated carbon offers a large surface area for palladium dispersion. Pd/C performs effectively in hydrogenation reactions and fine chemical synthesis. Its high porosity and stability support catalyst reuse.
- Carbon Nanotubes (Pd/CNT): Carbon nanotubes provide a larger surface area than activated carbon and exhibit notable electrical conductivity. Their high thermal and chemical stability makes them suitable for fuel cell and sensor applications.
2. Alumina (Al2O3)
- Palladium on Alumina (Pd/Al2O3): Alumina is used as a support because of its high thermal stability and its ability to withstand high-temperature reactions. Pd/Al2O3 is frequently employed in catalytic reforming and dehydrogenation processes, where high temperature resistance is critical.
- Gamma-Alumina: This form of alumina is known for its large surface area and adjustable acidic properties, which can be tailored for specific reactions such as selective oxidation or hydrogenation.

3. Silica (SiO2)
- Palladium on Silica (Pd/SiO2): Silica provides a large surface area and is used in reactions that require a neutral support. Pd/SiO2 is effective in processes such as hydrogenation and dehydrogenation of organic compounds. Its stability and low acidity render it suitable for procedures where acidic support interactions are not desired.
4. Zeolites
- Palladium on Zeolites (Pd/Zeolith): Zeolites have an ordered porous structure. Their acid–base characteristics and shape selectivity determine their catalytic properties. Pd/Zeolith catalysts are used in selective hydrogenation and hydrocracking, where pore size and shape govern catalytic activity.
- Hierarchical Zeolites: These zeolites contain both micropores and mesopores. They offer improved accessibility for larger molecules and increased stability, particularly in reactions such as selective oxidation.
5. Metal Oxides
- Palladium on Titanium Dioxide (Pd/TiO2): Titanium dioxide is a stable and versatile support material. Pd/TiO2 is employed in hydrogenation and oxidation reactions. Its involvement in electron transfer and photocatalysis under UV light is well documented.
- Palladium on Zirconium Dioxide (Pd/ZrO2): Zirconium dioxide is used because of its high thermal stability. Pd/ZrO2 is suited for selective hydrogenation and oxidation, where the catalytic properties of palladium and the thermal stability of zirconium dioxide are significant.
6. Magnesia (MgO)
- Palladium on Magnesia (Pd/MgO): Magnesium oxide supports palladium in reactions where both basic and thermal properties are crucial. Pd/MgO catalysts are useful in catalytic hydrogenation and reforming processes.
7. Graphene
- Palladium on Graphene (Pd/Graphene): Graphene, with its extensive surface area, electrical conductivity and mechanical strength, is increasingly used as a support for palladium. Pd/Graphene catalysts perform effectively in hydrogenation and fuel cell applications and provide enhanced electronic properties and activity.
8. Polymer Supports
- Palladium on Polymers (Pd/Polymer): Polymers such as polystyrene or polyethylene serve as supports for palladium in specific reactions, often in liquid-phase catalysis. These supports provide selectivity and stability in catalytic processes involving organic solvents.
9. Metallic Supports
- Palladium on Gold (Pd/Au): Gold is used as a support for palladium in some cases because of its specific electronic properties. Pd/Au catalysts are effective in particular oxidation and hydrogenation reactions, in which the gold surface enhances palladium activity.
- Palladium on Copper (Pd/Cu): Copper supports palladium in reactions that require a balance of oxidation and reduction properties. Pd/Cu catalysts are commonly employed in hydrogenation and selective oxidation processes.
Palladium-based Catalysts on Various Supports
|
Support Material |
Examples |
Key Properties |
Applications |
|
Carbon-based Supports |
Pd/C, Pd/CNT |
High surface area, stability, conductivity (CNT), porosity |
Hydrogenation, fine chemical synthesis, fuel cells, sensors |
|
Alumina (Al2O3) |
Pd/Al2O3, Gamma-alumina |
High thermal stability, adjustable acidic properties |
Catalytic reforming, dehydrogenation, selective oxidation |
|
Silica (SiO2) |
Pd/SiO2 |
Neutral support, stability, low acidity |
Hydrogenation, dehydrogenation of organic compounds |
|
Zeolites |
Pd/Zeolith, Hierarchical zeolites |
Ordered porous structure, acid–base characteristics, shape selectivity |
Selective hydrogenation, hydrocracking, selective oxidation |
|
Metal Oxides |
Pd/TiO2, Pd/ZrO2 |
Stability, electron transfer (TiO2), high temperature stability (ZrO2) |
Hydrogenation, oxidation, photocatalysis |
|
Magnesia (MgO) |
Pd/MgO |
Fundamental properties, thermal stability |
Hydrogenation, reforming processes |
|
Graphene |
Pd/Graphene |
Large surface area, electrical conductivity, mechanical strength |
Hydrogenation, fuel cells |
|
Polymer Supports |
Pd/Polymer |
Selectivity, stability in organic solvents |
Liquid-phase catalysis |
|
Metallic Supports |
Pd/Au, Pd/Cu |
Specific electronic properties (Au), balanced redox characteristics (Cu) |
Oxidation, hydrogenation, selective oxidation |
This table presents the various support materials used for palladium-based catalysts, their specific properties and respective applications. Further information is available at Stanford Advanced Materials (SAM).
Conclusion
The selection of the support material for palladium catalysers plays a crucial role in determining the catalyst’s effectiveness, stability and selectivity in various reactions. By optimising the interaction between palladium and its support, researchers and engineers can improve reaction efficiency and expand the scope of palladium catalysis in modern chemical processes.
Reference:
[1] Roman M. Mironenko, Olga B. Belskaya, Tatiana I. Gulyaeva, Mikhail V. Trenikhin, Vladimir A. Likholobov, Palladium nanoparticles supported on carbon nanoglobules as efficient catalysts for obtaining benzocaine via selective hydrogenation of ethyl 4-nitrobenzoate, Catalysis Communications, Volume 114, 2018, Pages 46–50, ISSN 1566–7367, https://doi.org/10.1016/j.catcom.2018.06.002.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us





 Chin Trento
Chin Trento



