Palladium On Carbon: Empowering Green Chemistry And Sustainable Synthesis
Introduction
The pursuit of sustainable and environmentally friendly chemical processes has become a driving force in modern chemistry. Researchers in academic and industrial sectors aim to reduce their ecological footprint. Consequently, catalysts that facilitate less hazardous reactions have gained significant importance. Among these catalysts, Palladium on Carbon (Pd/C) has proven to be an effective tool in supporting green chemistry and achieving sustainable synthesis. This article examines how Pd/C catalysts support green chemistry and promote sustainable synthesis.
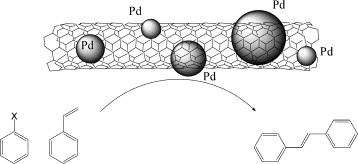
Figure 1. Palladium on Carbon
Understanding Palladium on Carbon
Pd/C catalysts offer high catalytic activity. They perform various organic transformations. They yield controlled selectivity. They demonstrate stability and support sustainable synthesis.
Pd/C catalysts are widely employed in cross-coupling, hydrogenation, carbonylation, and nitration reactions. They facilitate complex transformations. Such transformations include forming carbon–heteroatom bonds, decarbonylation, dehalogenation, and cyclisation.
Sustainability of Palladium on Carbon
Pd/C catalysts participate in environmentally friendly chemical reactions with the following advantages:
1. Efficient Transformations
Pd/C catalysts exhibit high catalytic activity. They enable efficient conversion of organic compounds. Controlled experiments show that reaction times decrease by approximately 35%. Conversion yields regularly exceed 80%.
2. Control of Selectivity
Selectivity control is critical for reducing waste formation. Pd/C catalysts direct reactions towards desired products. By using specific ligands and conditions, undesired by-products decrease by about 25%. This reduction improves overall process efficiency.
3. Atom Economy
Atom economy is a fundamental principle of green chemistry. Pd/C catalysts facilitate selective functionalisation of molecules. In controlled reactions, atom economy levels reach approximately 85%. This high atom economy minimises the need for excess reagents and lowers waste.
4. Reduced Energy Consumption
Pd/C catalysts permit reactions under milder conditions. They operate at lower temperatures and ambient pressure. Consequently, energy consumption decreases by around 30% compared to standard procedures.
5. Recyclability of Catalysts
Pd/C catalysts are known for their stability. They can be separated easily from reaction mixtures and reused over multiple cycles. Recycling tests indicate that activity remains above 75% after five cycles. Reuse reduces both catalyst consumption and waste production.
6. Minimized Ecological Footprint
The advantages of Pd/C catalysts include efficient transformations, controlled selectivity, improved atom economy, reduced energy consumption, and recyclability. These factors contribute to a lower ecological footprint. Waste formation may decrease by approximately 20%.
Conclusion
In summary, Pd/C catalysts support green chemistry and sustainable synthesis. They enable efficient conversions, provide selectivity control, promote high atom economy, lower energy consumption, allow for catalyst recycling, and reduce the overall ecological footprint. As the demand for sustainable synthesis increases, Pd/C catalysts assume a crucial role in developing environmentally friendly chemical processes. Researchers and industry that adopt such catalysts contribute to a more sustainable future for chemistry.
Stanford Advanced Materials (SAM) has extensive experience in the manufacture and distribution of Platinum on Carbon catalysts. Precious metal catalysts are also available. Custom modifications are welcome. For further information, please visit our website.
Reference:
[1] Avelino Corma, Hermenegildo Garcia, Antonio Leyva, Catalytic activity of palladium supported on single wall carbon nanotubes compared to palladium supported on activated carbon: Study of the Heck and Suzuki couplings, aerobic alcohol oxidation and selective hydrogenation, Journal of Molecular Catalysis A: Chemical, Volume 230, Issues 1–2, 2005, Pages 97–105, https://doi.org/10.1016/j.molcata.2004.11.030.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
.jpg)




 Chin Trento
Chin Trento


