Innovations In Optics: The Role Of GGG, SGGG, And NGG Garnet Boules
1 Preface
The widespread deployment of 5G networks opens new opportunities in the fibre optic communication market. Fibre optics remains the only medium capable of achieving the high data transmission rates required. As the demand for fibre increases as a consequence of 5G, the need for fibre optic isolators also grows. Fibre optic isolators are used in conjunction with doped fibre amplifiers to enhance overall amplification and reduce noise levels. In the rapid development of Dense Wavelength Division Multiplexing (DWDM) fibre optic systems, high-speed and high-performance configurations play an important role. In this context, optical isolators are particularly significant.
Gadolinium-Gallium Garnet (GGG), Scandium-Gadolinium-Gallium Garnet (SGGG) and Neodymium-Gallium Garnet (NGG) are frequently employed in various magneto-optical devices owing to their notable magneto-optical properties. These materials are increasingly adopted in the field of optical materials.

Figure 1 Fibre Optics
2 GGG
2.1 Introduction
Gadolinium-Gallium Garnet (GGG, formula Gd3Ga5O12) is a synthetic crystalline material with a garnet-like structure that is normally colourless. It has a cubic crystal lattice, a density of 7.08 g/cm³ and a Mohs hardness between 6.5 and 7.5. As an important raw material for optical devices, GGG exhibits unique properties. It possesses a relatively high refractive index and demonstrates good transparency in the visible spectrum, thereby allowing light transmission while retaining its optical characteristics. This material is suitable for the production of optical devices such as high-refractive-index lenses, optical components and laser systems. In addition, it exhibits several nonlinear optical effects, including the optical Kerr effect and self-focusing. GGG has low thermal conductivity and, given its effective heat dissipation, is well suited for use in optical devices and substrates. Most importantly, GGG demonstrates distinct magneto-optical behaviour, as evidenced by the Faraday spin effect, which has led to its application in magneto-optic storage and deflection systems.

Figure 2 Flaky GGG Crystals
2.2 Characteristics
Transistors and integrated circuits are fabricated on the surface of a semiconductor wafer, which in this context serves as the substrate (chip). The semiconductor substrate is important both for its electrical properties and its function in providing mechanical support.
As a substrate material, GGG exhibits properties that are well suited for substrate applications:
1. Structural compatibility between substrate and epitaxial film: Both the epitaxial material and the substrate should share the same or a closely matching crystal structure, with minimal lattice constant mismatch, high crystalline quality and a low defect density. Given that the lattice constant and thermal expansion coefficient of single-crystal GGG match those of YIG, single-crystal GGG is considered a suitable substrate for YIG and related magneto-optical epitaxial layers. These YIG and YIG-like materials have been applied in optical isolators, optical waveguides and integrated optics.
2. Matching of the thermal expansion coefficients between substrate and epitaxial film: A small difference in the thermal expansion coefficients is crucial. A large discrepancy may not only deteriorate the quality of the epitaxial film but also adversely affect the device during operation due to heat-induced stresses.
3. Chemical stability: The substrate material should exhibit good chemical stability and be capable of protecting the epitaxial film while maintaining its own integrity during processing.
4. Simplicity of fabrication and material cost: For use in mass production, the process of fabricating the substrate must be straightforward and cost efficient.
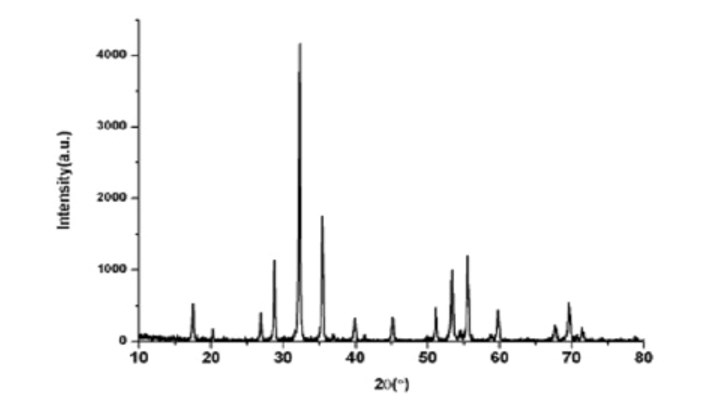
Figure 3 XRD Pattern of GGG at 1 000°C
2.3 Fabrication
The majority of magnetic bubble memories are manufactured on Gadolinium-Gallium Garnet substrates (GGG). These substrates act not only as carriers but also as seeds for the epitaxial growth of the magnetic storage layers. Any defect in the substrate will be replicated in the epitaxial layer, and therefore, the substrate must be highly uniform. Consequently, the preparatory process for GGG must be carefully controlled. The most common method for producing GGG is the pulling method, whose two critical parameters are temperature control and pull rate.
1. Temperature control: Maintaining precise control of the melt temperature is essential in the crystal growth process via the pulling method. The temperature distribution within the melt must preserve the melting point at the solid–liquid interface and ensure that the melt surrounding the seed crystal is maintained in an undercooled state while the bulk melt remains overheated. Consequently, the growth interface must continuously progress into regions of lower temperature, away from the isothermal surface at the freezing point, thus enabling crystal growth. In addition, the melt temperature is substantially higher than room temperature, thereby necessitating a continuous supply of heat.
2. Pull rate: The pull speed largely determines the speed and quality of crystal growth. An appropriate rotation speed ensures good mixing of the melt and reduces the radial temperature gradient, thereby preventing complications arising from undercooling. The standard pull rate is between 6 and 15 mm per hour.
Furthermore, during the growth of GGG, faint white particles may occasionally form within the crystals, which adversely affects their optical performance. The technical causes of this phenomenon and potential improvements are under investigation.

Figure 4 Occasional Faint White Spots in GGG Crystals
2.4 Applications (in Cryogenics)
Magnetic materials change the direction of their magnetic moments when exposed to an applied magnetic field. This process involves a change in magnetic entropy, leading to a thermal exchange. With the construction of an appropriate magnetic cooling system, it is possible to cool an object whilst absorbing heat. In cryogenic applications, GGG has been successfully employed at temperatures below 20 K for He-II flow and as a pre-cooling medium in helium–nitrogen liquefaction systems.

Figure 5 Columnar GGG Crystals
3 SGGG&NGG
3.1 SGGG
Scandium–Gadolinium–Gallium Garnet crystals (SGGG, formula Gd3Sc2Ga3O12) are produced by substituting a portion of Ga³⁺ in GGG crystals with Sc³⁺. They exhibit a similar structure and appearance, and are manufactured using the same method. SGGG offers several advantages:
1. In common with GGG, high-quality, coreless SGGG crystals are straightforward to grow and can avoid the impurities and stresses that may occur on small surfaces.
2. The presence of scandium in the garnet results in improved thermal conductivity and stable physico-chemical properties, which enhances heat dissipation and effectively avoids issues associated with surface overheating.
|
Materials |
GGG |
SGGG |
|
|
Chemical Formula |
Gd3Ga5O12 |
Substituted GGG |
|
|
Lattice Constant |
12.383 Å |
12.497 Å |
|
|
Density (g/cm³) |
7.13 |
7.09 |
|
|
Melting Point (℃) |
1725 |
1730 |
|
|
Mohs Hardness |
8.0 |
7.5 |
|
|
Refractive Index |
1.954 at 1064 nm |
1.954 at 1064 nm |
|
|
Orientation |
(111) (110) (100) |
(111) (110) (100) |
(111) |
Table 2 Comparison of the Properties of GGG, SGGG and NGG
3.2 NGG
Neodymium–Gallium Garnet crystals (NGG) are fabricated by replacing a portion of Ga³⁺ in GGG crystals with Nd³⁺. Their benefits are primarily as follows:
1. The crystal is relatively straightforward to grow, achieving growth rates up to 5 mm/h.
2. The crystal can be grown with a flat interface and with minimal stress concentrations and impurities. This facilitates the fabrication of large-area wafers for high-performance crystal applications.
3. In Yttrium-Aluminium Garnet (YAG) crystals, Nd has a distribution coefficient of between 0.1 and 0.2. In contrast, in GGG crystals the distribution coefficient of Nd is higher, reaching up to 0.52. This facilitates the growth of highly concentrated doped laser crystals and thereby increases the pumping efficiency [1].
4. When compared with Nd-doped glass, which is commonly used as a high-performance laser amplification medium, Nd:GGG crystals exhibit higher mechanical strength and improved thermal conductivity, thereby enabling more rapid cooling [2].
5. The homomorphic substitution of Nd³⁺ for Gd³⁺ effectively prevents the fragmentation of the luminescence levels in Nd³⁺ lasers [2].
6. The laser efficiency of Nd:GGG crystals is twice that of Nd-doped glass, a frequently used high-performance laser amplification medium, and may be utilised as the working medium in strategic short-range laser systems with powers of up to 100 kW [3,4].
|
Materials |
GGG |
SGGG |
NGG |
|
Chemical Formula |
Gd3Ga5O12 |
Substituted GGG |
Nd3Ga5O12 |
|
Lattice Constant |
12.383 Å |
12.497 Å |
12.509 Å |
|
Diameter |
1'', 2'', 3'' or 4'' |
1'', 2'', 3'' or 4'' |
|
|
Density (g/cm³) |
7.13 |
7.09 |
~7.4 |
|
Refractive Index |
1.954 at 1064 nm |
1.954 at 1064 nm |
~1.97 at 1064 nm |
|
Orientation |
(111) (110) (100) |
(111) (110) (100) |
(111) |
Table 2 Comparison of the Properties of GGG, SGGG and NGG
4 Conclusion
GGG, SGGG and NGG are utilised in laser technology, optical devices, magnetic applications and various high-technology fields due to their well-defined optical properties. GGG, which has been extensively studied and implemented, has found application in laser resonant cavities, magneto-optical devices and laser crystals. SGGG and NGG, benefitting from the doping with scandium and neodymium respectively, offer enhanced properties and are subjects of ongoing research into new applications.
References
[1] ZIMIK K, CHAUHAN R R, KUMAR R, et al. Study on the growth of Nd³⁺:Gd₃Ga₅O₁₂ (Nd:GGG) crystal by the Czochralski technique under different gas flow rates and using different crucible sizes for flat interface growth [J]. Journal of Crystal Growth, 2013, 363 (3): 76–79.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators




 Chin Trento
Chin Trento



