Titanium Dioxide Polymorphs: Rutile Vs. Anatase
Introduction
Titanium dioxide (TiO₂) is a compound widely used in industry and is valued for its distinct optical, physical and chemical properties. It occurs naturally in three polymorphic forms: anatase, rutile and brookite. Of these, anatase and rutile are the most important for industrial applications, whereas brookite is rarely employed due to its instability. This article outlines the principal differences between anatase and rutile and details their structures, properties and applications.
 [1]
[1]
Crystal Structure and Stability
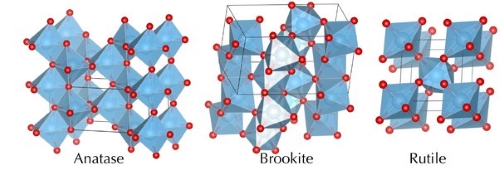
Anatase and rutile both belong to the tetragonal crystal system. They differ in lattice structure and stability.
- Anatase: This polymorph possesses an open crystal structure with a unit cell comprising 4 TiO₂ molecules. Its lattice is less compact, resulting in a lower density. Anatase is stable at room temperature but converts at higher temperatures, typically at 730 °C, into a more stable phase. This conversion is irreversible and exothermic, thereby indicating the higher thermodynamic stability of the final phase.
- Rutile: It exhibits a denser and more compact crystal structure; each unit cell contains 2 TiO₂ molecules. This dense arrangement results in a higher density and enhanced stability. It is the thermodynamically most stable form of TiO₂, and both anatase and brookite convert to this stable phase upon heating.
Physical Properties
--Density and Hardness
The relative density of anatase is between 3.8 and 3.9 g/cm³, and its Mohs hardness measures between 5.5 and 6.0. Given its lower density and hardness, anatase is less resistant than rutile.
With a relative density of 4.2 to 4.3 g/cm³, rutile is denser and more compact. Its Mohs hardness falls between 6.0 and 7.0, thereby making it more suitable for applications that require greater durability and wear resistance.
--Dielectric Constant
The dielectric constant of anatase is approximately 48, which is considerably lower than that of rutile. This lower dielectric constant restricts its use in applications that demand high dielectric properties.
Rutile has a much higher dielectric constant, averaging around 114. Given its stability, this property makes it appropriate for use in electronic applications.
Optical Properties
--Refractive Index
The refractive index determines a material’s ability to bend light, and TiO₂ is known for its comparatively high refractive index, which benefits optical applications. The refractive index of anatase is about 2.55, which, although high, is lower than that of rutile.
Rutile exhibits an even higher refractive index at approximately 2.71, thereby making it more effective in applications that require maximum light scattering and opacity.
-Light Scattering
The light scattering capacity of TiO₂ is critical for its role as a pigment in paints, coatings and other materials. Despite its adequate light scattering properties, anatase is less effective because of its lower refractive index.
Owing to its higher refractive index, rutile demonstrates enhanced light scattering, thereby increasing opacity and brightness in applications such as paints and coatings. Consequently, it is the preferred option for white pigments.
Electrical Properties
-Conductivity
Titanium dioxide is a semiconductor whose electrical conductivity depends on temperature and oxygen vacancies. Generally, anatase exhibits lower electrical conductivity and is less sensitive to temperature variations.
In contrast, the electrical conductivity of rutile increases markedly with temperature. At around 420 °C, its conductivity can rise by several orders of magnitude, thereby making it valuable for electronic components such as ceramic capacitors. This sensitivity to temperature and oxygen content renders it useful for sensor applications.
Applications
Both anatase and rutile are employed in various applications according to their respective properties.
1. Anatase
- Photocatalysis: Anatase is used in photocatalytic applications due to its higher reactivity under UV light. It effectively degrades organic contaminants and is therefore suitable for air and water purification systems, self-cleaning surfaces and antimicrobial coatings.
- Solar Cells: Due to its photoactive properties, anatase is employed in dye-sensitised solar cells to improve efficiency.
2. Rutile
- Pigments: Its high refractive index and excellent light scattering capacity make it ideal for use as a white pigment in paints, plastics and paper.
- Optical Components: Owing to its high refractive index, it is utilised in the manufacture of optical components such as lenses and coatings.
- Electronics: Given its elevated dielectric constant and increased electrical conductivity at higher temperatures, rutile is suitable for electronic devices, including capacitors and varistors.
- High-Temperature Applications: Its stability at elevated temperatures makes it appropriate for use in ceramic glazes, refractory materials and other high-temperature applications.
Quick Facts about Rutile and Anatase
|
Property |
||
|
Density (g/cm³) |
3.8 - 3.9 |
4.2 - 4.3 |
|
Mohs Hardness |
5.5 - 6.0 |
6.0 - 7.0 |
|
Dielectric Constant |
48 |
114 |
|
Refractive Index |
2.55 |
2.71 |
|
Light Scattering |
Moderate |
High |
|
Electrical Conductivity |
Lower, less sensitive to temperature variations |
Higher, increases with temperature |
|
Common Applications |
Photocatalysts, solar cells, paper, printing inks, textiles, rubber, ceramics, cosmetics |
Coatings, air purification, military applications, cosmetics, paints, and plastic articles |
Stanford Advanced Materials (SAM) provides high-quality titanium products at competitive prices. We supply both anatase and rutile forms of titanium dioxide, with options to meet your specific requirements. Please contact us for further information or to request a quotation.
Conclusion
Understanding the differences between anatase and rutile is vital for optimising their use in various industrial applications. Anatase is suitable for environmental and self-cleaning technologies due to its higher photocatalytic activity. Rutile, on the other hand, offers higher stability, density and optical properties, making it appropriate for pigments, coatings and electronic components.
The choice between these two materials depends on the specific requirements of the application. By utilising the distinct properties of these TiO₂ polymorphs, the industry can enhance the performance and efficiency of its products.
Reference:
[1] Stawarz, Sylwester & Witek, Natalia & Kucharczyk, Wojciech & Bakar, Med & Stawarz, Magdalena. (2019). Thermisch schützende Eigenschaften von Polymerkompositen mit Nano-Titandioxid. International Journal of Mechanics and Materials in Design. 15. 10.1007/s10999-018-9432-7.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Chin Trento
Chin Trento


