Comparison of Hot-Pressed Boron Nitride (HPBN) versus Pyrolytic Boron Nitride (PBN)
1 Introduction
Boron Nitride (BN) is a ceramic material that is employed in numerous industrial and technological sectors owing to its combination of properties. Its characteristics include high thermal conductivity, excellent electrical insulation, stability at temperatures above 2 000°C in inert atmospheres, chemical inertness towards most molten metals and corrosive media, a low dielectric constant with a small loss tangent, and inherent lubricity. These properties enable its use in single‐crystal growth crucibles, thermal management components in semiconductor fabrication, high‐temperature molten metal handling in metallurgy, and applications under extreme conditions in aerospace, nuclear energy and electronic packaging. The material is therefore widely adopted across many critical applications.
The final form—whether bulk, coating or film—and the main performance parameters (including purity, density, anisotropy, mechanical strength, and directional thermal/electrical conductivity) depend greatly on the production processes used. Among several manufacturing methods, hot-pressed boron nitride (HPBN) and pyrolytic boron nitride (PBN) are the two most important technologies for producing bulk material and thick-film BN. Although based on the same fundamental material, these processes differ fundamentally in their principles (physical sintering versus chemical vapour deposition), operating conditions (high-temperature/high-pressure consolidation versus atmospheric/low-pressure vapour-phase reactions) and resulting material characteristics. Consequently, the microstructures, property profiles and application domains vary between the two. This review presents a systematic comparison of the scientific principles, processing procedures, technical merits/limitations and differences in material performance—including aspects such as purity, density, thermal/electrical anisotropy, mechanical behaviour and vacuum characteristics—between HPBN and PBN. The work provides engineers and designers with a clear theoretical framework and practical guidelines for informed material selection and process understanding, tailored to specific technical requirements.

Fig. 1 Various Boron Nitride Ceramic Products
2 Detailed Process Principles and Workflow
2.1 Hot-Pressed Boron Nitride – HPBN
The production of hot-pressed boron nitride (HPBN) starts with high-purity hexagonal boron nitride (h-BN) powder. In order to improve sintering densification, minor quantities of sintering aids—typically boron oxide (B₂O₃), calcium oxide (CaO) or aluminium oxide (Al₂O₃)—are added to the powder. The process involves placing the homogenised powder into a graphite die designed for this purpose, followed by the simultaneous application of elevated temperatures (1 700–2 000°C) and uniaxial pressure (10–40 MPa) under an inert atmosphere (usually nitrogen, N₂, or argon, Ar) or vacuum conditions.
Densification occurs through several interacting mechanisms: (1) thermal softening of h-BN particles at high temperature; (2) pressure-induced particle sliding, rearrangement and plastic deformation; and (3) the formation of a liquid phase from the sintering aids at high temperatures. This liquid phase promotes densification by means of a dissolution-reprecipitation mechanism at particle surfaces and grain boundaries. The overall workflow involves accurate powder blending, die loading, control of the atmosphere (using vacuum pumping or gas purging), simultaneous ramping of temperature and pressure, an isothermal-isobaric hold period to complete densification, followed by carefully controlled cooling and depressurisation before the material is removed from the die. Although hot pressing produces near-net-shape components, subsequent machining (e.g. cutting, grinding) is often necessary to achieve the final dimensional tolerances.
HPBN products are generally produced as high-density bulk materials in forms such as plates, rods, crucibles, nozzles and insulating components. The available geometries and dimensions are limited by the design and load capacity of the graphite tooling used.
2.2 Pyrolytic Boron Nitride – PBN
Pyrolytic boron nitride (PBN) is manufactured using chemical vapour deposition (CVD). Gaseous precursors such as boron trichloride (BCl₃) or boron tribromide (BBr₃) are reacted with ammonia (NH₃). The process takes place in a specialised deposition furnace operated at temperatures between 1 400°C and 1 900°C under pressures that range from low vacuum to atmospheric. The sequence begins with loading a cleaned substrate (commonly high-purity graphite) into the reaction chamber, then vacuum pumping and the introduction of a precisely controlled mixture of precursor and carrier gases. Once the desired deposition temperature is reached via a programmed heating cycle, the precursor gases thermally decompose and recombine on the heated substrate surface. The resulting surface reaction follows the stoichiometry: BCl₃ + NH₃ → BN + 3HCl.
The growth mechanism is dominated by surface reactions in a layer-by-layer manner. Gaseous molecules adsorb on the substrate, migrate, nucleate and ultimately form BN crystalline structures through chemical bonding. The density of the deposition layer, its crystallographic orientation and the rate of growth are influenced by four key parameters:
- Substrate temperature (which modulates surface reaction kinetics and atomic mobility)
- Reactor pressure (which determines the mean free path of the gas molecules)
- Precursor gas flow ratios (which affect reaction equilibrium and impurity levels)
- Substrate surface condition (including roughness and crystallographic orientation that impact nucleation density)
After controlled cooling, the final products are categorised as either free-standing structures—separated from sacrificial substrates through mechanical or chemical release—or as conformal coatings deposited directly onto functional components. PBN offers ultra-high purity (>99.99%) and a non-porous microstructure, with typical morphologies including:
- Complex curved coatings (typically <500 μm in thickness)
- Thin-walled self-supporting structures (such as tubes, crucibles, or boats with wall thicknesses on the millimetre scale)
- Enclosed geometries with intricate three-dimensional profiles
Due to the slow growth rate inherent to the deposition process, the time required increases significantly for thicknesses above 5 mm. This renders the PBN process less economically viable for applications that require large volumes compared to bulk moulding techniques, such as hot pressing.
Fig. 2 Changes in The Mechanical Properties and Microstructure of Boron Nitride Blocks at Different Forming Temperatures
3 Core Process Characteristics and Comparison
3.1 Process Essence and Raw Material System
HPBN (Hot-Pressed Boron Nitride):
This method employs solid-state sintering. It uses hexagonal boron nitride (h-BN) powder and applies high temperature and pressure to achieve densification. Sintering aids (for example, B₂O₃ or CaO) assist by forming a liquid phase that reduces grain-boundary energy. This facilitates particle rearrangement and plastic flow.
PBN (Pyrolytic Boron Nitride):
This method is based on chemical vapour deposition (CVD). Gaseous precursors (BCl₃ or BBr₃) react with NH₃ in a controlled environment. The atomic-level deposition achieved through the surface chemical reaction (BCl₃ + NH₃ → BN + 3HCl) obviates the need for mechanical compaction.
3.2 Key Process Parameters
Table 1 Key Process Parameters Comparison of HPBN and PBN
|
Parameter |
HPBN (Hot-Pressed Boron Nitride) |
PBN (Pyrolytic Boron Nitride) |
|
Temperature Range |
1 700–2 000°C (dominated by solid-state diffusion) |
1 400–1 900°C (dominated by surface reactions) |
|
Pressure Conditions |
10–40 MPa (uniaxial mechanical pressure) |
Low vacuum to atmospheric pressure (no externally applied pressure) |
|
Atmosphere Control |
N₂/Ar inert atmosphere or vacuum |
Precisely controlled precursor and carrier gas mixture |
|
Time Scale |
Hours (during the holding stage) |
Days (due to a slow deposition rate for thickness buildup) |
3.3 Mechanism of Microstructure Formation
HPBN:
Densification is achieved by the following physical processes:
- Particle sliding and rearrangement under high pressure
- Grain-boundary diffusion assisted by elevated temperature
- Liquid-phase assisted dissolution-reprecipitation enabled by the sintering aids
The final product is a polycrystalline aggregate with grain sizes of approximately 5–20 μm.
PBN:
The growth is controlled by chemical kinetics through these steps:
- Adsorption of gas-phase molecules onto the substrate
- Surface migration and nucleation, which are highly sensitive to temperature
- Chemical bonding that forms a layered structure
The resulting product exhibits highly aligned columnar grains that extend through the thickness of the deposited layer.
Fig. 3 XRD Patterns and Microstructure of The Bulk Ceramics Prepared Through SPS.
3.4 Product Performance and Geometric Characteristics
Table 2 Comparison of Hot-Pressed (HPBN) and Pyrolytic Boron Nitride (PBN) Properties
|
Property |
HPBN (Hot-Pressed Boron Nitride) |
PBN (Pyrolytic Boron Nitride) |
|
Purity |
99.5–99.9% (with residual sintering aid impurities) |
>99.99% (produced by vapour deposition without additional impurities) |
|
Density |
1.8–2.0 g/cm³ (with some residual microporosity) |
2.2 g/cm³ (approximating theoretical density, pore-free) |
|
Formability |
Restricted to simple shapes determined by the mould design |
Suitable for complex curved coatings and free-standing thin-walled structures |
|
Typical Thickness |
From millimetres up to centimetres (no inherent thickness limitation) |
Coatings: <500 μm |
|
Anisotropy |
Low (due to random grain orientation) |
High (with the c-axis predominantly perpendicular to the substrate) |
3.5 Technical and Economic Comparison
HPBN offers a cost-effective solution for the mass production of thick-section components, such as crucibles and insulating plates. The capital equipment requirements are lower when using hot pressing rather than CVD systems. In contrast, PBN allows for the production of highly pure, contamination-free components, which is essential for many semiconductor applications. Its method of near-net-shape fabrication permits the production of complex thin-walled structures. However, HPBN is limited in the production of sub–1 mm thin-walled components because of the risk of brittle fracture during demoulding, while PBN can become economically unviable for sections thicker than 5 mm, given its deposition rate of approximately 20 μm/h.
Table 3 Comparative Fabrication Processes: Hot-Pressed Boron Nitride (HPBN) Sintering vs. Pyrolytic Boron Nitride (PBN) Chemical Vapour Deposition
|
Comparison Dimension |
Hot-Pressed Boron Nitride (HPBN) |
Pyrolytic Boron Nitride (PBN) |
|
Process Category |
Solid-state sintering |
Chemical Vapour Deposition (CVD) |
|
Raw Material Form |
h-BN powder with sintering additives |
BX₃ (where X = Cl or Br) with NH₃ gas precursors |
|
Densification Mechanism |
Application of mechanical pressure (10–40 MPa) |
Surface chemical reaction energy |
|
Core Temperature Range |
1 700–2 000°C |
1 400–1 900°C |
|
Microstructure Formation |
Particle rearrangement and grain-boundary diffusion |
Sequence of adsorption, migration, nucleation and bonding |
|
Key Equipment |
Hot-pressing furnace |
Vacuum CVD reactor |
|
Product Purity |
99.5–99.9% |
>99.99% |
|
Geometric Capabilities |
Thick monolithic blocks (≥1 cm) |
Complex curved coatings |
|
Thickness Limitations |
Minimum ~1 mm (due to brittleness constraints) |
Maximum ~5 mm (for economic feasibility) |
|
Typical Applications |
Molten metal crucibles, high-temperature insulators |
Semiconductor chamber liners, MBE source boats |
4 Comparative Analysis of Material Properties
Although both Hot-Pressed Boron Nitride (HPBN) and Pyrolytic Boron Nitride (PBN) belong to the hexagonal boron nitride system, the differences in their microstructures result in distinct macroscopic properties. HPBN, produced by high-temperature/high-pressure sintering, exhibits a polycrystalline structure with randomly oriented grains and contains isolated closed pores (0.5–3 μm), leading to densities in the range 1.8–2.0 g/cm³. The presence of residual sintering aids limits the purity to approximately 99.5–99.9%. In contrast, PBN is produced using vapour deposition. It forms columnar grains that are oriented normal to the substrate. The structure is fully dense (2.20–2.25 g/cm³) and primarily consists of a single phase that exceeds 99.99% purity, as no sintering additives are introduced.
4.1 Thermal and Electrical Properties
PBN’s columnar structure results in significant anisotropy in thermal conductivity. Along the deposition plane (a–b plane), thermal conductivity reaches between 150 and 220 W/(m·K), values close to those determined theoretically for h-BN single crystals. Conversely, the thermal conductivity in the direction perpendicular to the deposition plane (c-axis) is reduced to approximately 1–3 W/(m·K), thereby acting as a thermal barrier. HPBN exhibits a more uniform, isotropic thermal conductivity in the range 25–60 W/(m·K) due to its random grain orientation, with grain boundaries contributing to additional phonon scattering. Both materials provide good electrical insulation with volume resistivities over 10¹³ Ω·cm. In addition, the pore-free structure of PBN leads to marginally higher electrical breakdown strengths (40–50 kV/mm) compared to those of HPBN (30–40 kV/mm).
4.2 Vacuum and Chemical Stability
The fully dense nature of PBN enables an extremely low outgassing rate in ultra-high vacuum environments, measured at approximately 5×10⁻¹¹ Torr·L/(s·cm²) (for pressures <10⁻¹⁰ mbar). This is one to two orders of magnitude lower than the outgassing rate observed for HPBN. In terms of chemical resistance, both materials withstand corrosion from molten metals (e.g. aluminium, copper, gold) and non-oxidising acids. However, PBN exhibits enhanced resistance to molten alkali (for instance, NaOH) due to the absence of impurity phases. Oxidation tests indicate that PBN remains stable in dry air up to 850°C, while HPBN begins to oxidise at approximately 800°C. This is attributed to the presence of impurities at the grain boundaries in HPBN, which facilitate oxidation.
4.3 Mechanical Properties and Machinability
HPBN exhibits a flexural strength in the range 30–100 MPa and fracture toughness between 2.5 and 3.5 MPa·m¹/². These properties allow for the use of conventional machining techniques, including turning and drilling. In contrast, PBN shows higher strength (120–180 MPa) in the a–b plane. However, its layered structure results in brittle behaviour along the c-axis, with a fracture toughness of only 1.0–1.8 MPa·m¹/². Its microhardness, measured at 350–400 kgf/mm², is approximately 1.5 times higher than that of HPBN. Due to the simultaneous presence of high hardness and low fracture toughness, machining of PBN by conventional turning or milling is impractical. Typically, PBN components require precision grinding or are produced directly through deposition methods.
Table 4 Comparison of Key Performance Parameters
|
Property |
Hot-Pressed Boron Nitride (HPBN) |
Pyrolytic Boron Nitride (PBN) |
|
In-Plane Thermal Conductivity |
25–60 W/(m·K) |
150–220 W/(m·K) |
|
Through-Thickness Thermal Conductivity |
25–60 W/(m·K) |
1–3 W/(m·K) |
|
Vacuum Outgassing Rate |
Approximately 10⁻⁹ Torr·L/(s·cm²) |
<5×10⁻¹¹ Torr·L/(s·cm²) |
|
Machinability |
Appropriate for turning and drilling |
Restricted to cutting and grinding |
4.4 The Physical Essence of Performance Differences
The notable anisotropy in PBN is a direct result of its highly oriented columnar grain structure. Strong covalent bonds within the a–b planes support efficient thermal conduction, whereas the weak van der Waals forces along the c-axis result in limited thermal conduction. In contrast, HPBN’s random grain stacking leads to uniform properties. The presence of isolated micropores in HPBN introduces a slight compromise in mechanical strength while permitting conventional machining. The coexistence of high in-plane strength and low through-thickness toughness in PBN reflects the inherent characteristics of hexagonal boron nitride, in which covalent bonding in the basal planes is significantly stronger than the interlayer van der Waals interactions.
4.5 Engineering Selection Principles
In semiconductor ultra-high vacuum systems, the PBN crucible is used due to its low outgassing performance and high purity. For applications that require improved mechanical load-bearing capability or complex geometries (for example, molten salt electrolysis cell linings), HPBN offers distinct processing advantages. These differences can be attributed to the precision of structural control: HPBN represents the performance limits inherent in traditional powder metallurgy, whereas PBN demonstrates the ability of vapour deposition to achieve near-intrinsic material properties. Future advancements will need to address the limitations of the PBN process or enhance the in-plane thermal conductivity of HPBN by controlling grain orientation.

Fig. 4 Pyrolytic Boron Nitride VGF Crucible
5 Typical Application Scenario Analysis
5.1 Advantageous Fields of Hot-Pressed Boron Nitride (HPBN)
HPBN is suited for applications where isotropic properties, machinability and cost efficiency are required. It is used in large high-temperature containers, such as GaAs/GaP semiconductor melt crucibles (with diameters exceeding 300 mm) and aluminium alloy casting distribution pans. Its uniform thermal expansion coefficient (3.5–4.5×10⁻⁶/°C) ensures that it withstands thermal cycling stresses, while thick components (≥20 mm) provide adequate containment of molten materials. For components with complex functionality, HPBN is used to machine cooling channels in plasma arc chamber linings. It is also applicable in the manufacture of glass moulding dies, achieving a surface finish of Ra 0.4 μm to minimise adhesion. In applications where cost is a significant constraint, such as welding nozzles or heat treatment fixtures, the sintered production method for HPBN reduces costs by 60–80% compared to PBN, while keeping porosity under 3% to maintain performance. A practical example includes the insulation supports for polycrystalline silicon ingot furnaces, which maintain a flexural strength of ≥80 MPa and show less than 0.5 mm deformation after 2 000 hours at 1 560°C. These supports offer a measurable improvement over alternative materials such as graphite.
PBN is applied in high-end scenarios where purity is critical. In ultra-high-purity semiconductor manufacturing, PBN is used for MBE source boats to ensure minimal metal contamination (Al, Fe). Vertical Bridgman (VB) GaAs crystal growth utilises PBN crucibles to attain carbon impurity levels below 1×10¹⁵ atoms/cm³. Other applications in ultra-high vacuum systems, such as synchrotron beamline collimators (10⁻¹⁰ Pa), use PBN liners to reduce hydrocarbon desorption. In mass spectrometer ion sources, the low sputtering yield (less than 10⁻⁴ atoms/ion) of PBN is an advantage. Additionally, thermal management systems such as laser diode heat sinks employ 2 mm thick PBN substrates to deliver in-plane conductivity exceeding 200 W/(m·K) while limiting conduction along the c-axis to approximately 1.5 W/(m·K). PBN's near-net-shape capability allows for the production of thin-walled structures, such as RF plasma generator tubes with thicknesses in the range of 0.8–1.2 mm and an as-deposited Ra less than 0.1 μm, thus ensuring operational reliability. In an ion implanter case study, the use of PBN arc chambers (purity 99.995%) reduced the level of metal contamination on silicon wafers to 5×10⁹ atoms/cm², which is approximately two orders of magnitude lower than levels observed with alumina components.
Table 5 Selection Decision Mechanism
|
Selection Dimension |
HPBN Preferred Conditions |
PBN Preferred Conditions |
|
Purity Requirement |
Up to 99.9% is acceptable |
>99.99% is required to avoid trace contamination |
|
Vacuum Level |
High vacuum (HV, 10⁻³–10⁻⁷ Pa) |
Ultra-high/extreme vacuum (UHV/XHV, <10⁻⁸ Pa) |
|
Thermal Management |
Suitable for isotropic heat distribution |
Requires engineered anisotropy with a difference of more than 100× between the a–b plane and the c-axis |
|
Geometric Complexity |
Three-dimensional complex structures that require machining |
Thin-walled shells or tubular structures that are formed by direct deposition |
|
Cost Constraint |
Medium to low budget |
High performance justifies higher cost |
5.2 Deep Interconnectivity of Application Scenarios
Intrinsic Purity Advantage:
The high purity achieved in PBN arises from its vapour deposition process, which does not introduce elements beyond boron and oxygen. In contrast, sintering aids in HPBN may incorporate up to 0.1% B₂O₃, which can volatilise above 1 400°C and lead to contamination in epitaxial layers such as those of InP.
Thermal Management Innovation:
PBN’s engineered anisotropy permits improved thermal management. For example, in kilowatt-class laser modules, the use of PBN substrates has been shown to increase heat dissipation efficiency by 300% and restrict the temperature increase in thermally sensitive optical components to less than 5°C, in comparison to typical aluminium nitride substrates that often result in temperature rises exceeding 30°C.
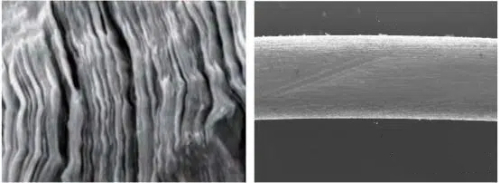
Fig. 5 The CVD Process Also Yields PBN A Nearly Perfect Layered Structure, As Shown in The Figure Below. This Results in Anisotropic Thermal Conductivity—The Thermal Conductivity in The Deposition Direction (A-Direction) And Perpendicular to The Deposition Plane (C-Direction) Differs by A Factor of About 20, Making It an Ideal Material for Manufacturing Crystal Growth Crucibles. Therefore, PBN Crucibles Are Also a Popular Choice in The Field of GaAs Crystal Growth.
Failure Mode Mitigation:
In applications such as plasma arc chambers, HPBN is used due to its uniform wear properties. The polycrystalline structure yields similar erosion rates in all directions (0.1–0.3 mm per 1 000 hours), whereas PBN may exhibit layer-by-layer delamination under ion bombardment due to its anisotropic structure.
6 Conclusion
The differences in performance between Hot-Pressed Boron Nitride (HPBN) and Pyrolytic Boron Nitride (PBN) arise from the distinct manufacturing processes employed. HPBN, produced via powder sintering, results in a polycrystalline structure with isotropic properties and acceptable machinability at a lower cost. However, the presence of residual porosity (0.5–3%) and sintering additives limits its purity to near 99.9% and results in a higher vacuum outgassing rate (approximately 10⁻⁸ Torr·L/(s·cm²)). In contrast, PBN, produced by chemical vapour deposition, forms columnar grains with a density approaching 2.20–2.25 g/cm³ and achieves a purity exceeding 99.995%, along with outgassing rates below 5×10⁻¹¹ Torr·L/(s·cm²). Its thermal conductivity is highly anisotropic, with in-plane values up to 220 W/(m·K) and through-thickness values around 2 W/(m·K), although the weak interlayer bonding results in limited machining options.
Application selection is determined by the following performance requirements:
- Vacuum Requirements: PBN is required for pressures lower than 10⁻⁸ Pa
- Thermal Management: PBN is necessary when directional thermal conductivity is needed (with in-plane conductivity above 200 W/(m·K) and minimal conduction through the thickness)
- Cost Considerations: PBN is selected when performance requirements justify higher production cost and limited machinability
Future research should address the inherent limitations of each process. HPBN requires improvements to increase its in-plane thermal conductivity (currently under 40 W/(m·K)), while PBN needs enhancements in c-axis toughness. Hybrid structures, such as depositing PBN coatings on HPBN substrates, have already demonstrated a 50% increase in operational lifetime for semiconductor carrier trays under controlled conditions.
Ensuring a consistent supply of high-quality, application-specific materials remains essential. Stanford Advanced Materials (SAM) is a leading supplier in this sector, offering a broad range of boron nitride products that meet the requirements of the semiconductor, aerospace and industrial sectors. In addition to product supply, SAM provides technical support and customised solutions to assist material engineers and researchers in optimising performance and advancing their work.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us



 Chin Trento
Chin Trento



