Carbon: Element Properties And Uses
Carbon is a non-metal element that plays a crucial role in life processes, industrial applications, and chemistry. It has distinct chemical and physical properties that make it highly versatile.

Introduction to the Element
Carbon, symbolised as "C" on the periodic table, is a chemical element with atomic number 6. It is one of the most abundant elements on Earth, found in both living organisms and the Earth's crust. Its ability to form stable bonds with many elements, including itself, makes it essential to life as we know it.
Carbon occurs naturally in various forms, primarily as graphite, diamond, and amorphous carbon (such as coal). It is also a critical component of organic molecules, which are the building blocks of life. Carbon's remarkable bonding capabilities allow for the creation of a vast array of compounds, making it central to organic chemistry.
Chemical Properties Description
Carbon exhibits a wide range of chemical properties. One of the key features is its ability to form covalent bonds with a variety of other elements, including hydrogen, oxygen, nitrogen, and itself. This makes it highly versatile in the formation of different types of molecules. Here are some key chemical properties of carbon:
- Bonding Behaviour: Carbon can form single, double, or triple bonds, depending on the type of bond it shares with other elements.
- Reactivity: While carbon is not highly reactive in its elemental form (as graphite or diamond), it reacts with oxygen at high temperatures to form carbon dioxide (CO₂). It can also react with halogens, hydrogen, and metals.
- Oxidation States: Carbon can exhibit oxidation states of -4 (as in methane, CH₄), 0 (as in graphite or diamond), +2 (as in carbon monoxide, CO), and +4 (as in carbon dioxide, CO₂).
- Allotropes: Carbon exists in several allotropes, such as diamond, graphite, graphene, and fullerenes, each with different chemical properties due to the arrangement of carbon atoms.
Physical Properties Data Table
|
Property |
Value |
|
Atomic Number |
6 |
|
Density |
2.267 g/cm³ (diamond), 1.550 g/cm³ (graphite) |
|
Melting Point |
Sublimates at around 3 915°C (diamond) |
|
Boiling Point |
Sublimates at around 3 915°C (diamond) |
|
Hardness |
Very hard (diamond), soft (graphite) |
|
Electrical Conductivity |
Conducts electricity (graphite, graphene) |
|
Thermal Conductivity |
Good conductor (diamond) |
|
Colour |
Colourless (diamond), grey/black (graphite) |
For more information, please check Stanford Advanced Materials (SAM).
Common Uses
Carbon is used in a wide variety of industries due to its diverse physical and chemical properties:
- Steel production: Carbon is a key component in steel manufacturing, where it is used to modify the properties of iron to make it stronger and more durable.
- Carbon black: Used as a black pigment in inks, paints, and coatings.
- Electronics: Graphite is used in electronic components like batteries, capacitors, and as an electrode material.
- Filtration: Activated carbon is used in water and air filtration systems to remove impurities.
- Lubricants: Graphite's lubricating properties make it useful in reducing friction in mechanical systems.
- Diamond: Used in cutting tools and jewellery, diamond is the hardest known material.

Preparation Methods
Carbon can be obtained through various methods, depending on the desired form:
- From coal: Carbon is extracted from coal through a process called "carbonisation," which involves heating coal to high temperatures in the absence of oxygen to produce coke, a pure form of carbon.
- Graphite: Naturally occurring graphite is mined, and artificial graphite can be produced through the high-temperature treatment of carbon-containing materials like petroleum coke.
- Diamond: While diamonds occur naturally, synthetic diamonds can be manufactured using high-pressure, high-temperature methods or chemical vapour deposition (CVD).
- Activated Carbon: Produced by heating carbon-rich materials, such as wood or coconut shells, in the presence of gases to create a porous material with high surface area.
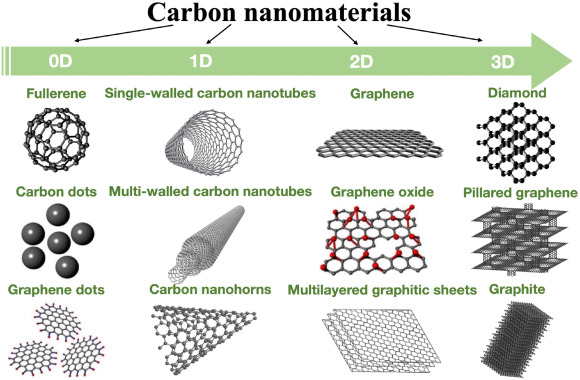 [1]
[1]
Related Industrial Products
Carbon is central to many industrial processes and products:
- Carbon composites: Used in aerospace, automotive, and sports industries for lightweight and strong materials.
- Carbon nanotubes: These are used in electronics, energy storage devices, and nanotechnology applications due to their strength and conductivity.
- Carbon dioxide (CO₂): Widely used in the production of soft drinks, fire extinguishers, and as a refrigerant.
Frequently Asked Questions
What is carbon's most common form?
Carbon is most commonly found in the form of graphite, diamond, and amorphous carbon (such as coal).
Is carbon toxic?
Carbon itself is not toxic in its elemental form, but carbon monoxide (CO), a gas produced by the incomplete combustion of carbon-containing fuels, can be highly toxic.
Why is carbon important for life?
Carbon is the backbone of all organic molecules, including proteins, lipids, and nucleic acids, making it essential for life processes.
What are the uses of activated carbon?
Activated carbon is used in filtration systems, such as water and air purifiers, to remove impurities and toxins.
How is diamond different from graphite?
While both are made entirely of carbon atoms, diamond has a crystal structure that makes it the hardest material on Earth, whereas graphite has layers of carbon atoms arranged in a planar structure that allows it to be soft and slippery.
Reference:
[1] Kuan Cheng, Samuel Wallaert, Haleh Ardebili, Alamgir Karim, Advanced triboelectric nanogenerators based on low-dimension carbon materials: A review,
Carbon, Volume 194, 2022, Pages 81-103, ISSN 0008-6223, https://www.sciencedirect.com/science/article/pii/S0008622322002093

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us




 Chin Trento
Chin Trento



