Common Reaction Types Of Homogeneous Precious Metal Catalysts
Introduction
Noble metals such as platinum, palladium, rhodium and gold are frequently employed as homogeneous catalysts due to their high catalytic activity, selectivity and stability. They are also noted for their high thermal stability and chemical inertness, thereby rendering them effective catalysts. Because of these characteristics, homogeneous noble metal catalysts serve a wide range of applications in the pharmaceutical, petrochemical, chemical and materials science sectors.
In this article, we describe the common reaction types of homogeneous noble metal catalysts. We aim to provide a clearer understanding of these catalysts.

Figure 1. Noble Metal Catalysts
Common Reaction Types of Homogeneous Noble Metal Catalysts
Homogeneous noble metal catalysts are applied in a variety of reactions. Typical examples include hydrogenation, hydroformylation reactions, coupling reactions, etc.
-Hydrogenation:
Hydrogenation is a reaction in which hydrogen is added to unsaturated organic compounds, usually with the aid of a catalyst. Homogeneous catalysts such as platinum and palladium are frequently employed in hydrogenation reactions to convert alkenes into alkanes and nitro compounds into amines.

Figure 2. Metal-catalysed hydrogenation and dehydrogenation reactions for efficient hydrogen storage [1]
--Dehydrogenation:
Dehydrogenation is the reverse of hydrogenation, in which hydrogen is removed from a molecule. Noble metal catalysts such as platinum and rhodium are employed in dehydrogenation reactions to produce alkenes from alkanes and carbonyl compounds from alcohols.
-Oxidation:
In oxidation reactions, a molecule loses electrons. Homogeneous noble metal catalysts are used to convert alcohols into aldehydes or ketones and alkenes into epoxides. Among these oxidation reactions, the "Hoechst-Wacker" process is the most well‐known, whereby acetaldehyde is synthesised from ethene and oxygen using Pd/Cu catalysts in aqueous, chloride‐containing solutions.
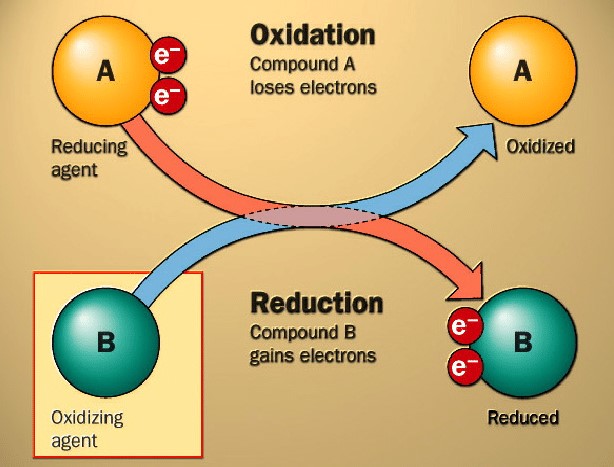
Figure 3. Basic oxidation and reduction [2]
-Reduction:
Reduction is the opposite of oxidation, whereby a molecule gains electrons. Generally, these homogeneous catalysts are applied in reduction reactions to convert nitro compounds into amines and carbonyl compounds into alcohols.
--Coupling:
In coupling reactions, two or more molecules are joined to form a larger molecule. Catalysts such as palladium and platinum are employed in coupling reactions to form carbon–carbon bonds, as demonstrated in the Suzuki reaction and the Heck reaction.
--Carbonylation:
Carbonylation refers to reactions that form aldehydes, ketones or similar compounds using carbon monoxide (CO). The most recognised method is the carbonylation of methanol into acetic acid. This method is also known as the Monsanto process. All these processes cannot be performed without the use of rhodium catalysts.
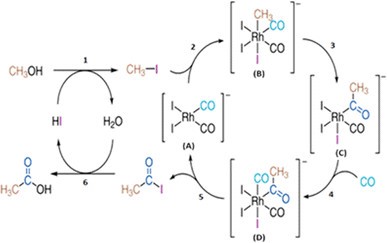
Figure 4. Proposed catalytic cycles for the rhodium-catalysed methanol carbonylation reaction (Monsanto process) [3]
-Hydroformylation:
Hydroformylation, also known as oxo synthesis, is a process in which alkenes are converted into aldehydes using a mixture of carbon monoxide (CO) and hydrogen (H2). Rhodium catalysts replaced earlier cobalt catalysts in these procedures.
-Isomerisation:
Isomerisation is a reaction in which a molecule undergoes structural rearrangement. Platinum and rhodium are typical homogeneous catalysts used in isomerisation reactions to convert alkanes into branched alkanes and alkenes into isomers.
The table below provides further information comparing various reaction types of homogeneous noble metal catalysts.
Table 1. Various Reaction Types of Homogeneous Noble Metal Catalysts
|
|
Definition |
Examples |
|
Hydrogenation |
Addition of hydrogen; |
Conversion of alkenes into alkanes and of nitro compounds into amines; |
|
Dehydrogenation |
Removal of hydrogen; |
Conversion of alkanes into alkenes and of alcohols into carbonyl compounds; |
|
Oxidation |
Loss of electrons; |
Conversion of alcohols into aldehydes or ketones and of alkenes into epoxides; |
|
Reduction |
Gain of electrons; |
Conversion of nitro compounds into amines and of carbonyl compounds into alcohols; |
|
Coupling |
Joining two or more molecules to form a larger molecule; |
The Suzuki reaction and the Heck reaction; |
|
Carbonylation |
Formation of aldehydes and ketones using carbon monoxide (CO); |
Monsanto process; |
|
Hydroformylation |
Conversion of alkenes into aldehydes using carbon monoxide (CO) and hydrogen (H2); |
Use of rhodium catalysts; |
|
Isomerisation |
Structural rearrangement; |
Conversion of alkanes into branched alkanes and of alkenes into isomers; |
Conclusion
In summary, homogeneous noble metal catalysts are applied in a range of chemical reactions, including hydrogenation, dehydrogenation, oxidation, reduction, coupling, carbonylation, hydroformylation and isomerisation. Their catalytic activity, selectivity and stability are well documented, thereby supporting advances in chemical processes within the pharmaceutical, petrochemical and fine chemical industries.
Stanford Advanced Materials (SAM) is a supplier of platinum catalysts, palladium catalysts and other noble metal catalysts. Please submit an enquiry if you are interested.
References:
[1] Shimbayashi, Takuya & Fujita, Ken-Ichi. (2020). Metal-catalysed hydrogenation and dehydrogenation reactions for efficient hydrogen storage. Tetrahedron. 76. 130946. 10.1016/j.tet.2020.130946.
[2] Azman, Nur & Ramli, Muhammad & Isa, Siti. (2019). A Review of hybridization of carbon nanotube into graphene for gas sensor application. IOP Conference Series: Materials Science and Engineering. 551. 012017. 10.1088/1757-899X/551/1/012017.
[3] Budiman, Anatta & Nam, Ji & Park, Jae & Mukti, Ryan & Chang, Tae & Bae, Jong Wook & Choi, Myoung. (2016). Review of Acetic Acid Synthesis from Various Feedstocks Through Different Catalytic Processes. Catalysis Surveys from Asia. 20. 10.1007/s10563-016-9215-9.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators





 Chin Trento
Chin Trento



