Common Sulfides And Their Applications
Introduction
Sulphides have played an important role in various industries for many years. These materials possess specific chemical and physical properties that qualify them for applications in catalysis, energy storage, electronics and other technical fields. This article discusses several frequently used sulphides and their applications.
1. Lithium Sulphide (Li₂S): A Key Component for Energy Storage
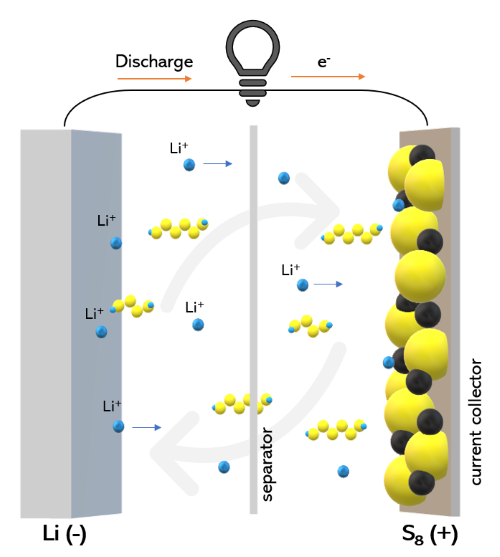
Lithium sulphide (Li₂S) is an essential component in lithium–sulphur battery technology, an energy storage solution with a high theoretical energy density. Lithium–sulphur batteries are able to store more energy than conventional lithium-ion batteries, making them suitable for electric vehicles and grid storage. Li₂S functions as the cathode material in these batteries, thereby enabling specific electrochemical processes for energy storage and release.
 [1]
[1]
Despite these advantages, lithium–sulphur batteries face challenges, including the shuttle effect whereby lithium polysulphides dissolve in the electrolyte and reduce the battery life. Researchers are developing methods to stabilise lithium sulphide in the battery, thereby advancing its market readiness.
2. Molybdenum Disulphide (MoS₂): A Versatile Catalyst and Lubricant
Molybdenum disulphide (MoS₂) is a versatile sulphide used both as a solid lubricant and as a catalyst. In its natural mineral form, molybdenite exhibits a layered structure that reduces friction in machinery and aerospace components. MoS₂ is also employed as a catalyst in hydrodesulphurisation, a process used in petroleum refining to remove sulphur impurities from crude oil. Its catalytic function derives from active edge sites that promote sulphur removal. Researchers are investigating its potential in water splitting reactions for hydrogen production.

3. Iron Sulphides (FeS and FeS₂): The Basis of Metallurgy and Related Applications
Iron sulphides, including iron(II) sulphide (FeS) and iron disulphide (FeS₂) (commonly known as pyrite), are used extensively in metallurgical processing, chemical synthesis and photovoltaic applications. In metallurgy, FeS is frequently a by-product that serves as a source of both sulphur and iron. Pyrite (FeS₂) is utilised in the production of sulphuric acid. The sulphur dioxide released during the roasting of pyrite is converted into sulphuric acid, which is used in fertiliser production and wastewater treatment. Moreover, FeS₂ exhibits semiconducting properties and is under investigation for use in solar cells, although further study is required to address issues of stability and conversion efficiency.
4. Zinc Sulphide (ZnS): A Key Material for Optical and Luminescent Applications
Zinc sulphide is employed in optical and display technologies as it remains transparent in the infrared range and emits light when stimulated. One common application of ZnS is as a phosphor in screens, luminous materials and X‐ray screens. When doped with small amounts of copper, ZnS phosphors emit light suitable for various applications, thereby supporting energy-efficient displays.
ZnS is also used in the manufacture of infrared optics, such as lenses and windows. Given its transparency in both the visible and infrared spectrums, ZnS is appropriate for use in night vision and thermal imaging technologies.
5. Cadmium Sulphide (CdS): Applications in Photovoltaics and Electronics
Cadmium sulphide (CdS) is an important semiconductor material used primarily in photovoltaic cells and various electronic applications. In solar cells, CdS is often paired with cadmium telluride (CdTe) to form a highly efficient photovoltaic layer. Its band gap property allows CdS to absorb sunlight effectively, making it a critical component in thin film solar cells.
Due to the toxicity of cadmium, environmental concerns have prompted research into safer alternatives. Nonetheless, CdS-based thin film solar technology remains competitive because of its high conversion efficiency, ease of production and scalability, thereby contributing to solutions for environmental issues.
6. Nickel Sulphide (NiS): A Catalyst in the Chemical Industry
Nickel sulphide (NiS) plays an important role as a catalyst in chemical processes, particularly in the hydrogenation of organic compounds. NiS catalyses reactions that add hydrogen to organic molecules, a process which is important in the manufacture of products such as margarine and specific pharmaceuticals. The material has demonstrated stability under rigorous reaction conditions, thereby qualifying as a durable catalyst. In addition, NiS is used in specialist glass and ceramics. In glass production, NiS particles can cause spontaneous fracture in tempered glass. Manufacturers are investigating methods to mitigate such occurrences, given its influence on glass behaviour.
7. Copper(I) Sulphide (Cu₂S): Conductive Films and Antibacterial Applications
Copper(I) sulphide (Cu₂S) is used in the electronics industry as a conductive material in thin films. Cu₂S films exhibit high electrical conductivity and are suitable for electronic devices that require transparent conductive films, such as touchscreens and other display technologies. Cu₂S also possesses antibacterial properties, which are beneficial in medical devices and coatings where bacterial resistance is necessary. Researchers are examining the potential of copper sulphide nanoparticles as antibacterial agents in healthcare to reduce infection rates and improve patient outcomes.
|
Sulphide |
Applications |
Key Properties |
|
Li–S batteries for electric vehicles and grid storage |
High energy density, rechargeable |
|
|
Lubricant in machinery, catalyst for fuel refining |
Reduces friction, stable catalyst |
|
|
Iron Sulphides |
Metallurgy, sulphuric acid production, solar cells |
Supplies sulphur, semiconducting |
|
Displays, infrared optics, phosphors |
Infrared transparent, phosphorescent |
|
|
Cadmium Sulphide |
Solar cells, electronics |
Light-absorbing, paired with CdTe |
|
Nickel Sulphide |
Chemical catalyst, glass ceramics |
Stable in reactions, affects tempered glass |
|
Copper(I) Sulphide |
Conductive films, antibacterial coatings |
High conductivity, antibacterial |
Conclusion
Sulphide materials offer a diverse range of applications. They are integral to energy storage, catalysis, electronics and optics. Innovations in battery technology, catalysis, photovoltaics and other fields utilise these compounds to improve efficiency and expand their application range. Further information is available at Stanford Advanced Materials (SAM).
Reference:
[1] Lithium–Sulphur Battery. (20/08/2023). In Wikipedia. https://en.wikipedia.org/wiki/Lithium%E2%80%93sulfur_battery

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators





 Chin Trento
Chin Trento



