Gas Diffusion Layers: Why Fibre Felts Are the Preferred Choice
Gas diffusion layers (GDLs) are critical elements in fuel cells and electrolyzers. They enable efficient transport of gases, electrons, and liquids between the flow channels and the catalyst. Fibre felts are the preferred option because they represent the ideal combination of conductivity, porosity, and flexibility.
What Are Gas Diffusion Layers?
Gas diffusion layers are porous materials placed between the catalyst layer and the flow field in electrochemical devices. Their primary function is to enable uniform delivery of reactant gases to the catalyst surface while facilitating efficient evacuation of product gases. In fuel cells, GDLs manage hydrogen and oxygen transport to maximise electrochemical reactions and minimise concentration losses. In electrolyzers, they assist in effectively evacuating hydrogen and oxygen gases generated at the electrodes.
Besides gas transport, GDLs also provide mechanical support for the catalyst layer and are involved in water management. Uniform distribution of water avoids flooding or drying of the catalyst, both of which can severely reduce performance. Electrical conductivity is also a significant function, whereby GDLs conduct electrons between the catalyst and external circuits. With such demands, the material choice of GDLs is essential to device efficiency and longevity in the long term.
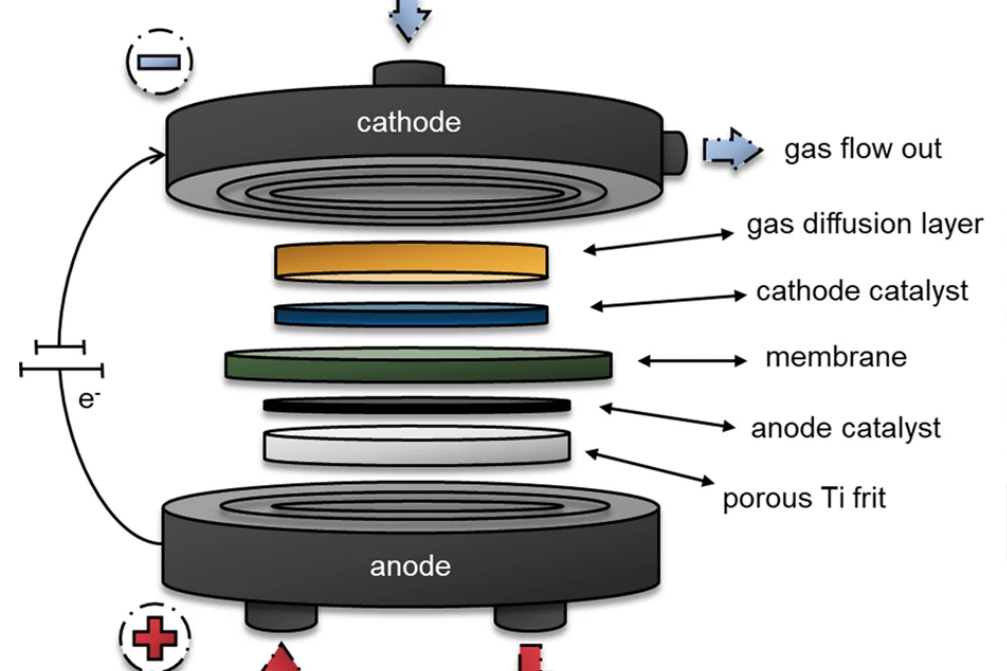
Fig. 1 Schematic illustration of the zero-gap electrolyser cell [1]
What Are Fibre Felts?
Fibre felts are nonwoven mats that are formed by entangling fibres into a highly porous network. They are typically made of carbon, which are characterised by their electrical conductivity and chemical stability. Fibres within a felt are randomly oriented, forming a three-dimensional structure that offers interconnected paths to gases and liquids. The structure differs from that of woven fabrics or carbon papers and imparts higher porosity and flexibility with maintained mechanical strength.
The production process of fibre felts binds the fibres through heating, pressure, or resin treatments to create mats with engineered thickness, density, and permeability. Carbon felts are exceptionally suited for electrochemical environments due to the fact that they are inert, high-temperature-resistant, and corrosion-resistant.
Why Are Fibre Felts Ideal for Gas Diffusion Layers?
Fibre felts offer a balance of properties that makes them extremely appropriate for use as GDLs. One of the most important properties is that they have high gas permeability and porosity, which allows reactant gases to be distributed evenly to the catalyst layer while product gases can be expelled effectively. This allows concentration polarization to be minimised and electrochemical efficiency to be high.
One other key advantage is excellent electrical conductivity. Carbon fibre felts provide low resistance paths for electrons, facilitating efficient current collection and distribution across the catalyst surface. Chemical stability is also significant; fibre felts are resistant to corrosion and degradation in aggressive acidic or alkaline environments, which are common in fuel cells and electrolyzers.
Mechanical flexibility is also an important factor. Felts composed of fibres can be compressed for close contact with the catalyst and flow field without cracking or degradation of structural integrity. Compressibility provides for thermal expansion and mechanical stresses during operation for long-term performance.
In fuel cells, they manage water. They spread water evenly, prevent flooding, and provide optimum catalyst hydration. In electrolyzers, they provide the rapid release of hydrogen and oxygen bubbles with minimal accumulation of bubbles, making high efficiency possible at high current densities.
Where to Use Fibre Felts for Gas Diffusion Layers?
Fibre felts have the biggest use in fuel cells and water electrolyzers, where water management and gas transport are crucial. In PEM fuel cells, fibre felts are employed as GDLs for uniform delivery of hydrogen and oxygen to the catalyst layer and for the free exit of water. In alkaline or PEM electrolyzers, fibre felts are employed for effective release of hydrogen and oxygen bubbles from the surfaces of electrodes in order to facilitate high current density operation. Besides these, the fibre felts are applied in unitised regenerative fuel cells, redox flow batteries, and other electrochemical reactors where uniform gas and liquid distribution, chemical and mechanical stability, are essential for high performance.
List of Fibre Felt Materials
Titanium, nickel, and stainless steel fibre felts each offer distinct advantages for gas diffusion layers. Titanium felt is prized in PEM electrolyzers and fuel cells for its high porosity, strength, and excellent resistance to acidic environments. With customizable size and porosity, it ensures stable performance and efficient gas release even at high current densities. Nickel felt, known for its conductivity and resistance to alkaline corrosion, is widely used on the cathode side of alkaline electrolyzers and in some fuel cells to promote hydrogen generation. Stainless steel felt, though less corrosion resistant, combines durability, wear resistance, and lower cost, making it a practical choice for large electrolyzers and flow batteries where mechanical strength is critical.
|
Feature / Material |
Titanium (Ti) |
Nickel (Ni) |
Stainless Steel (SS) |
|
Properties |
High porosity, strong acid resistance |
High conductivity, alkali resistant |
Strong, wear-resistant, lower cost |
|
Applications |
PEM electrolyzers (anode), fuel cells |
Alkaline electrolyzers (cathode), some fuel cells |
Large electrolyzers, flow batteries |
|
Advantages |
Stable at high current, corrosion proof |
Efficient hydrogen release |
Durable, economical alternative |
Conclusion
Gas diffusion layers contribute significantly to the performance and lifespan of fuel cells and electrolyzers. Among the prospective materials, fibre felts stand out for their high porosity, electrical conductivity, chemical stability, and mechanical flexibility. For more advanced materials, please check Stanford Advanced Materials (SAM).
Reference:
[1] Samu AA, Szenti I, Kukovecz Á, Endrődi B, Janáky C. Systematic screening of gas diffusion layers for high performance CO2 electrolysis. Commun Chem. 2023 Feb 24;6(1):41. doi: 10.1038/s42004-023-00836-2. PMID: 36828885; PMCID: PMC9958001.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us
 Chin Trento
Chin Trento

