The uses of Vanadium and its uses according to its oxidation states
This content is from a 2025 Stanford Advanced Materials College Scholarship submission by Addison Boyle.
Introduction
Vanadium is part of the transition metals; its atomic number is 23. It has an abundance in the Earth’s crust of approximately 0.02%, comparable to zinc.1 Despite its abundance, it did not receive significant attention until the past 50 years. Research indicated that vanadium could have a biological role through the vanadate ion.2 There are at least six different oxidation states; vanadium primarily exists in +3, +4, and +5. When isolated, each of the oxidation states exhibits a distinct colour. V4+ is blue, V3+ is green, and V2+ is violet.3 These oxidations are also the most relevant for biological systems.4
Vanadium in the Blood:
These oxidation states of vanadium are crucial as they can undergo redox reactions.4 The stability is influenced by ligation, the solvent, and the pH.2 Biogenically, this signifies that there are reducing agents that could convert vanadium to lower oxidation states.4 This process is pH dependent; as pH increases, the redox potentials decrease.4 When administered in drug form, it enters the bloodstream and interacts with the constituents of blood serum.4 The reaction involving vanadium determines the speciation of vanadium.1
Under mildly acidic conditions, V(III) resembles existing biological systems when formed.1 Blood plasma may contain reducing agents that could lead to the stabilization of V(III), allowing for the formation of V(III)2-hTF species.1 These species resemble Fe(III) 2-hTF, which is identified by cell receptors.1 This process could occur when V(III) or Fe(III) are attracted to hTF if V(IV)O is available in the blood plasma, thereby making a reduction to V(III) favourable from an energy gain perspective.1 The binding affinity of V(III) has a logK of 20, while V(IV)O+2 has a logK of 13.1 This reaction needs to occur under mildly acidic conditions.1
It has been observed that other oxidation states of vanadium (V(IV) and V(V)) can undergo ligand exchange and redox interconversion.1 Ligands with low molecular masses include lactate and citrate, while high molecular mass ligands comprise hTF, albumin, and immunoglobulin G.1 With hTF being the most predominant.1 Like with V(III), V(IV) and V(V) interact effectively due to the residues of the Fe(III) binding sites of hTF.1 Given that Fe(III) 2-hTF is recognised by cell receptors for endocytosis,1 it might also be possible for V(IV) and V(V) complexes to pursue a similar course.1 Incubating V(IV)OSO4 with apo-hTF has been shown to correspond to hTF.1 It is also observed that the formation ratio is lower than that of Fe(III) 2hTF.1 There have also been indications that VIVO and VIVO-carrier complexes, along with V(V) as monovanadate, may bind to holo-hTF, which is the transporting agent within the blood.1 This suggests that vanadium could be present in blood plasma and undergo endocytosis when holo enters the cells.1
Vanadate:
There are three primary types of reactions that can be undertaken with vanadate in aqueous chemistry: self-condensation reactions, coordination reactions, and redox reactions.4
In condensation reactions, vanadate anions are protonated by oligomers in a mildly acidic environment to favour their formation.4 This is vital for cellular function, given how pH affects different organelles.4 In tumour cells, it has been demonstrated that they exhibit elevated organelle pH.4 This suggests that vanadate could be utilised to identify tumour cells displaying elevated pH.4 This could involve a series of oligomers of vanadate in an acidic environment.4
Since vanadate shares similarities with phosphate, it has begun to be utilised in coordination reactions; however, it remains primarily in the testing phase, with most coordinated reactions being theoretical.4 Phosphate plays a significant role in biological systems and is involved in numerous biological recognition and bio-catalytic processes.1 Here, VO43- possesses structural and electronic similarity to PO43- (Fig. 1).4 Vanadate complexes exhibit five-coordinate trigonal-bipyramidal geometries, matching the five-coordinate transition states assumed to form during the reactions of phosphate-dependent enzymes (Fig. 1).1 While mono- and polydentate ligands can be employed, vanadate esters and vanadate anhydrides are primarily utilised due to their relationship with phosphate. 4 Through computer modelling, it was found that most vanadate existed in the forms of H2VO4- and HVO42- when proteins were excluded; however, upon including proteins, the majority of vanadium was bound to transferrin.4 Stabilising the metal complex of vanadate via ligands enhances the probability of reduction.4 This leads to a tendency for coordination reactions to reduce during biological processes.4
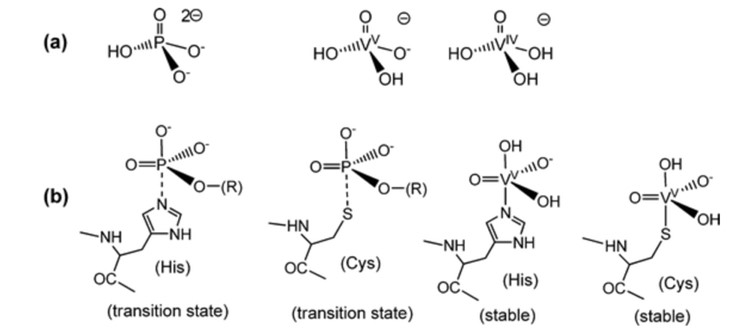
Fig. 1. Comparison of phosphates and vanadate with transition states and stability.5
In redox reactions within biological systems, the oxidation states of vanadium that are pertinent are +3, +4, and +5.4 The majority of reductions of vanadate occur from V(V) to V(IV).4 This type of reaction takes place with various biomolecules, particularly in organelles.4 It also serves as a buffer for phosphotyrosyl proteins.4 This results in intervention within the insulin-signalling pathway.4 The application in diabetes treatment arises as, once bonded at the enzyme’s active site, vanadate is not readily released like phosphate.1 This obstructs the site from phosphate, leading to inhibition of the enzyme.1 There are concerns regarding the use of vanadate for diabetes treatment, including vanadium-induced oxidative stress, global toxicity, impacts on the immune system, and inflammation.1
Cancer Research:
The properties of vanadium have been examined for their chemopreventive and anti-tumour effects in cancer research.1 Tests have primarily been in animal models and on malignant cell lines.1 Vanadocene dichloride and several peroxovanadates are the main vanadium compounds that have been employed.1 Vanadocene dichloride is soluble in water, and at a pH of 7, the chloride ions are substituted with H2O, leading to V(C5H5)2]2+.1 It has been observed that it binds to DNA, causing a variation in the behaviour of cisplatin.1 It has also exhibited limited toxicity, no transfer across the blood-brain barrier, and is not detected in the brain.1 The targets for the anti-cancer properties of vanadium include disruption of cellular metabolism, signal transduction pathways, and impairment of cell proliferation.1 Concerns have been raised regarding the varied responses and safety of vanadium, leading to a lack of clinical research.1 In light of these concerns, research continues.1 V(V)-mhcpe systems have been tested in vitro, demonstrating greater toxicity towards tumour cells compared to non-tumour cells.1 V(IV)-complexes containing 1-10-phenanthroline derivatives as ligands and V(IV)O-dppz complexes have exhibited anti-cancer properties (Fig. 2), particularly against leukaemia.1

Fig. 2. Depiction of anti-tumour of vanadate compounds, with buffer stability pH 7.1
Amavadin:
A species of fungus that accumulates vanadium is Amanita muscaria, or the fly agaric.6 A typical mushroom contains less than 0.5 mg of V kg-1, but A. muscaria can contain 100 mg V kg-1.6 The parts of the mushroom body that contain vanadium are the stipe, cap skin, cap flesh, gills, spores, and bulb.6 The highest concentration of vanadium is found in the bulb at 1 000 mg V kg-1.6 This part of the mushroom is located at the lowest part of the stipe, making it the most susceptible to contamination from the soil.6 When examining the topsoil, the median concentration of vanadium was 60 mg kg-1, with a range from 1.28 to 537 mg kg-1.6 The vanadium discovered in A. muscaria was negatively charged and was termed amavadin (Fig. 3) by Bayer and Kneifel.6 Electron paramagnetic resonance (EPR) spectroscopy revealed that the oxidation state of vanadium was +4.6 EPR spectra also indicated that the various parts of the mushroom contained the same compound, amavadin.6 This compound consists of two tetradentate ligands depicted in Fig. 3 that can coordinate to the vanadium centre, forming two isomers that appear in nearly equal mixtures in both synthetic and natural amavadin.6
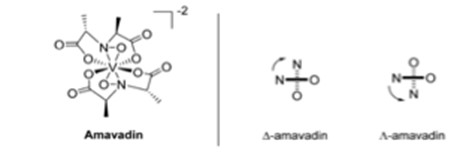
Fig. 3. Structure of amavadin, with isomers D and L.6
The oxidation of V(IV) to V(V) is reversible in amavadin, indicating potential catalytic properties.6 A broad range of reactions was catalysed by this complex.6 This led to the synthesis of various amavadin analogues incorporating different metal ion centres or modified ligands.6 Upon synthesising amavadin from A. muscaria, different vanadium complexes were detected, with vanadyl acetate being predominant, finding that the solid comprised 26% (w/w) vanadyl acetate and 74% (w/w) amavadin.6
Conclusion
In conclusion, vanadium has been shown to possess multiple applications within biological systems, ranging from cancer treatment to its presence in mushrooms. Research continues to investigate the different oxidation states and their reduction processes in biological systems, such as blood or cells. The primary oxidation states of vanadium are +3, +4, and +5. The ligands typically associated with vanadium are oxygen complexes, particularly vanadate and vanadyl acetate. Further investigation is warranted regarding vanadium and its implications for the scientific community.
References:
1. J.Costa Pessoa, Journal of Inorganic Biochemistry, 2015, 147, 4-24
2. A. Butler, and C. J. Carrano, Coordination Chemistry Reviews, 1991, 109, 61-65
3. D. Rehder, Bioinorganic Vanadium Chemistry, Wiley, Incorporated, New York, 2008.
4. X. Yang and K. Wang, in Progress in Molecular and Subcellular Biology, eds. W. E. G. Muller, P. Jeanteur, R. E. Rhoads, D. Ugarkovic, and M. R. Custodio. Springer, Manz, Germany, 2013, ch. 1, pp 1-19.
5. D. Rehder, Metallomics, 2015, 7, 732
6. S. Braeuer, M. Walenta, L. Steiner, and W. Goessler, Royal Society of Chemistry, 2021, 36, 954-967.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Dr. Samuel R. Matthews
Dr. Samuel R. Matthews


