The 2025 Nobel Prize in Chemistry: What Are MOFs?
The Royal Swedish Academy of Sciences awarded the 2025 Nobel Prize in Chemistry to Susumu Kitagawa, Richard Robson, and Omar M. Yaghi for their research on metal–organic frameworks (MOFs). The materials, with their large internal surface areas, adjustable pore structures, and unitary design, have proven themselves to be a significant component of materials chemistry with applications in energy storage, environmental decontamination, and molecular engineering.
Fig. 1 2025 Chemistry Nobel Prize
Introduction to MOFs
MOFs are three-dimensional solid crystals composed of metal ions or clusters coordinated with organic ligands, the latter generating three-dimensional structures with highly adjustable pore architectures. Due to the combination of high surface area, low density, and elastic structure, chemists can tailor frameworks with predictable pore size, chemical functionality, and mechanical properties.
Certain MOFs achieve an internal surface area of more than 7,000 m²/g, significantly better than activated carbon, with potential for molecule storage and separation. The modularity of MOFs also facilitates functionalisation for applications ranging from gas separation and storage to drug delivery and catalysis.
History and Development of MOFs
Construction of metal–organic frameworks (MOFs) began with Richard Robson in 1989, as he first proposed the theory by connecting copper ions with a four-armed organic linker to produce a crystalline network with accurately defined cavities. This initiated what became a rapidly developing field of research.
Subsequently, Susumu Kitagawa demonstrated the versatility of MOFs with structures' ability to alter through structural transformation, enabling frameworks to adjust according to guest molecules.
Omar Yaghi subsequently extended the field further with the synthesis of MOF-5, a material that possesses a surface area of greater than 3,000 m²/g and good gas uptake capabilities, illustrating the material's practical utility in real-world applications.
Their contributions collectively established MOFs as a family of porous crystalline solids with potential applications and scientific interest.
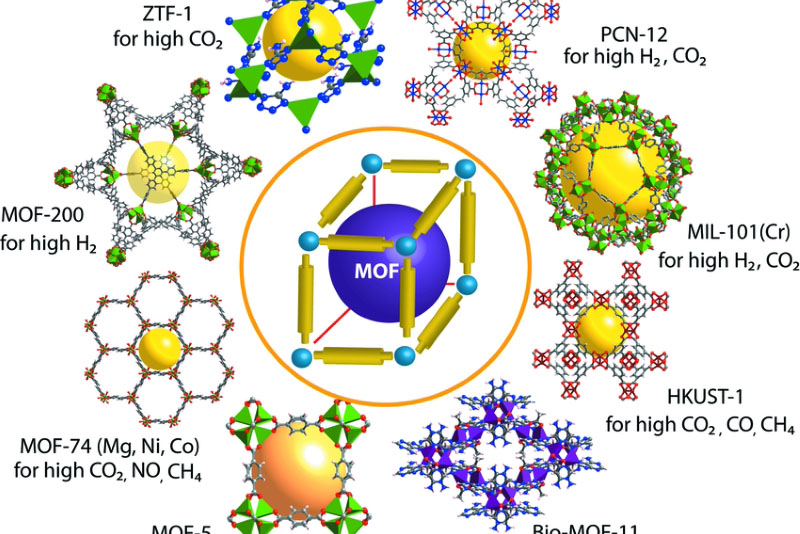
Fig. 2 Schematic representation of important reported MOFs
Synthesis Methods of MOFs
Solvothermal remains the most common method of MOF synthesis. In this process, metal salts and organic ligands are mixed in formamide-functionalised protic or aprotic organic solvents. The reaction is generally carried out under autogenous pressure above the solvent's boiling point in an autoclave, where crystal growth is permitted and orderly structures are obtained. Slow crystal growth is typically necessary to achieve large defect-free crystals with optimal internal surface area.
Even though solvothermal synthesis is conventional and reliable, several other methods have emerged to make product structures adjustable and enhance efficiency. Techniques such as microwave-assisted, sonochemical, mechanochemical, electrochemical, and ionothermal synthesis are increasingly being adopted.
For instance, mechanochemical synthesis utilises grinding and mechanical energy rather than solvents, reducing environmental burden and allowing for rapid framework development. Microwave-assisted synthesis has also been demonstrated to generate MOFs with comparable crystallinity within minutes rather than hours. All these developments are important for large-scale production of MOFs and determining new architectures.
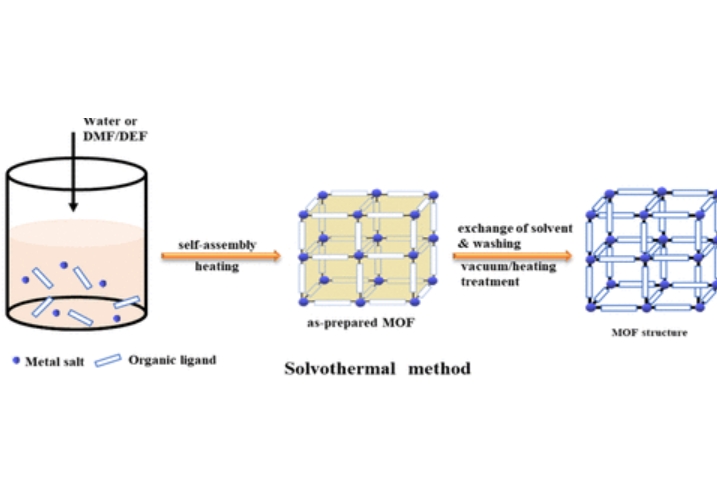
Fig. 3 Conventional Solvothermal Synthesis of MOF Structures
Potential Applications of MOFs
The distinctive MOF properties—low density, high surface area, porous yet adjustable porosity, and structural flexibility—allow for a wide range of potential applications:
- Gas Storage and Delivery: MOFs possess significant value in hydrogen, methane, and carbon dioxide storage. For example, MOF-5 adsorbs over 20 wt% hydrogen under 77 K and 1 bar, and MOF-177 has CO₂ adsorption of over 6 mmol/g at 298 K under 1 bar. These attributes position MOFs as materials for clean energy storage such as hydrogen fuel cells and methane vehicles.
- Environmental Remediation: MOFs have been employed to remove contaminants from water and air. Some MOFs selectively adsorb PFAS ("forever chemicals") from wastewater, while others have affinity for carbon dioxide, enabling carbon capture. For instance, Mg-MOF-74 has CO₂ adsorption capacities of up to 8 mmol/g at room conditions, which makes it viable for emission control applications.
- Water Harvesting: Certain MOFs can harvest water from arid air. Field tests in arid environments have shown that Zirconium-based MOF-801 can collect 2.8 litres of water per kilogram of MOF per day under low humidity (20–30% relative humidity).
- Drug Delivery: The porous architectures of MOFs enable encapsulation of therapeutic molecules for controlled release. Experimental studies have shown that MIL-100(Fe) matrices can release anti-cancer drugs with improved stability and targeted release characteristics, reducing systemic toxicity.
- Energy Storage and Electronics: MOFs are being investigated for applications in supercapacitors, batteries, and catalysis. MOFs can serve as high capacitance and conductivity electrode materials or as catalyst supports for catalytically active metal nanoparticles.
These uses demonstrate that MOFs are no longer merely laboratory curiosities; they are exhibiting quantifiable, real-world performance across numerous applications. Commercialisation on scales larger than the laboratory remains challenging, but research is ongoing to improve stability, reproducibility, and cost-effectiveness.
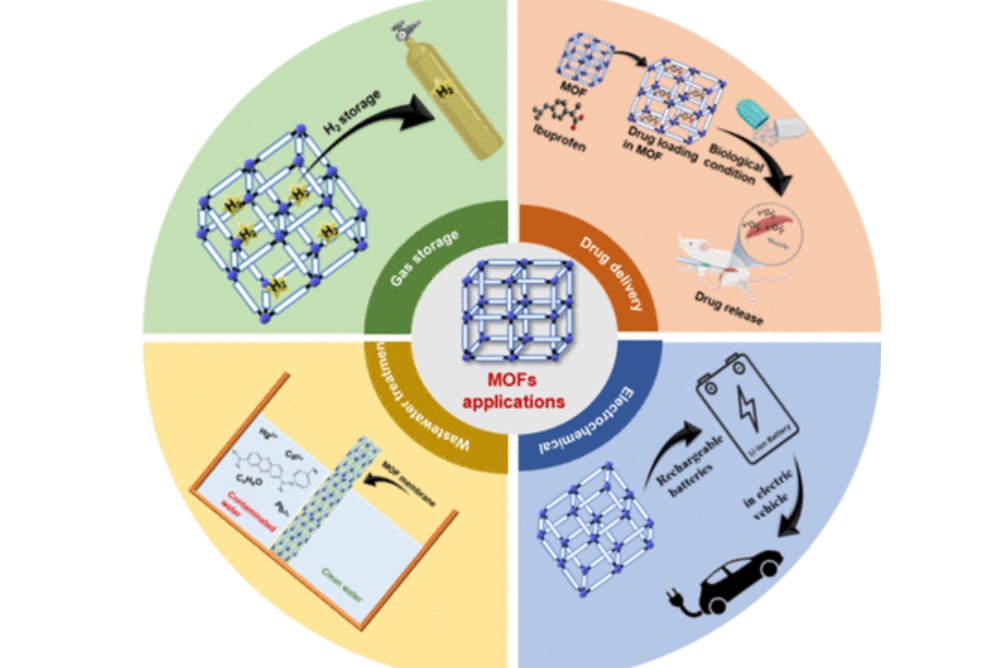
Fig. 4 Applications in Energy, Drug Delivery, and Wastewater Treatment
Conclusion
The 2025 Chemistry Nobel Prize awarded to Kitagawa, Robson, and Yaghi highlights the critical significance of MOFs. From structural concepts to synthesis methods and potential applications, MOFs exemplify the intersection of fundamental chemistry and practical utility. For further industry news and technical support, please visit Stanford Advanced Materials (SAM).
References:
- Dey, Chandan & Kundu, Tanay & Biswal, Bishnu & Mallick, Arijit & Banerjee, Rahul. (2013). Crystalline metal-organic frameworks (MOFs): synthesis, structure and function. Acta Crystallographica Section B. 70. 3-10. 10.1107/S2052520613029557.
- Ganesan, M. (n.d.). Are metal-organic frameworks (MOFs) at a commercial tipping point? CAS Insights.
- Raptopoulou, C. P. (2021). Metal-organic frameworks: Synthetic methods and potential applications. Materials (Basel), 14(2), 310. (https://doi.org/10.3390/ma14020310)
- Sanders, R. (2025, 8 October). UC Berkeley’s Omar Yaghi shares 2025 Nobel Prize in chemistry. Berkeley News.
- The Royal Swedish Academy of Sciences. (2025). The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry 2025. Nobel Prize Press Release.
- Yusuf, V. F., Malek, N. I., & Kailasa, S. K. (2022). Review on metal–organic framework classification, synthetic approaches, and influencing factors: Applications in energy, drug delivery, and wastewater treatment. ACS Omega, 7(49), 44507–44531. (https://doi.org/10.1021/acsomega.2c05310)

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us
 Dr. Samuel R. Matthews
Dr. Samuel R. Matthews


