GGG Vs. GGAG Vs. TGG Garnet Crystals: A Comparative Analysis
1 Introduction
Garnet crystals with a garnet structure are known for their exceptional thermal stability, tunable optoelectronic properties and versatile chemical adaptability. These materials have become important components in modern photonic technology. Among them, Gadolinium Gallium Garnet (GGG, Gd3Ga5O12), its aluminium‐substituted derivative (GGAG, Gd3Ga2Al3O12) and the terbium‐doped variant (TGG, Tb3Ga5O12) demonstrate different performance profiles that are determined by their individual elemental substitutions. GGG is employed in mid‐infrared laser systems and as epitaxial substrates because of its wide transparency and excellent lattice matching. GGAG benefits from aluminium‐induced lattice contraction which increases the thermal conductivity by 23% (up to 9,2 W/(m-K)) and improves resistance to radiation, making it a suitable material for high-power lasers and scintillators. TGG uses the strong magneto-optical response of Tb³⁺ to modify optical isolators used in fibre optic communication. However, a systematic comparison covering the principles of structural engineering, thermomechanical behaviour and application-specific photonic functions has not been sufficiently investigated, thereby leading to suboptimal material selection in emerging technologies such as quantum photonics and integrated optoelectronics. This study addresses the gap by correlating composition-induced structural variations (e.g. Al/Ga ratio, Tb3+ substitution) with measurable performance thresholds, thereby providing guidance for adapting garnet crystals to the varied requirements of optical systems.

Fig. 1 GGG Wafer
2 Background and Significance of the Study
2.1 Introduction to Garnets
Garnets are a group of silicate minerals derived from the Latin term "granatum". They have been used since the Bronze Age as gemstones and abrasives. Six common types of garnets are classified by their chemical composition. These include Pyrope, Almandine, Spessartine, Andradite, Grossular and varieties of Tsavorite, Hessonite and Chrysoberyl. Garnets form two series of solid solutions: (1) the Rhodolite–Ferroaluminium–Manganese–Aluminium garnet series and (2) the Chalkoklas–Calcium–Aluminium garnet series. Their general chemical formula is A3B2(SiO4)3, where A represents divalent ions (such as calcium, magnesium, iron, manganese, etc.) and B represents trivalent ions (including aluminium, iron, chromium, titanium, vanadium, zirconium, etc.). Frequently encountered are magnesium–aluminium garnet, ferro–aluminium garnet and manganese–aluminium garnet. Calcium‐based garnets generally contain a large divalent cation and are known as calcium–aluminium, calcium–iron and calcium–chromium garnets. Some garnets possess hydroxyl groups in their lattice forming hydrous variants such as hydrotalcite–aluminium garnet. The chemical composition is inherently complex due to extensive solid-solution substitution, so natural garnets generally represent intermediate compositions rather than end-member phases.

Fig. 2 Garnet Crystal
Garnets exhibit a typical cubic crystal system and their structures are island silicates consisting of isolated SiO4 tetrahedra that are connected by metal cations (e.g. Al3+, Fe2+, Mg2+). Single crystals typically develop in forms such as rhombic dodecahedra, tetragonal trisoctahedra, hexaoctahedra and their aggregates. These aggregates often occur as dense grains or blocks. The symmetry is associated with the space group Ia3̅ (or Ia3d) and the observed striations on crystal faces indicate periodic fluctuations in melt or solution composition during growth.
2.2 The Role of Garnets in Laser Technology, Magneto-optical Devices and Radiation Detection
Garnet crystals play an important role in laser technology. Their cubic crystal structure (space group Ia3̅ or Ia3d) and adjustable chemical compositions give rise to significant physical and optical properties. For example, in Neodymium‐doped Yttrium Aluminium Garnet (Nd:YAG), Nd3+ ions occupy the dodecahedral sites and, under the influence of the crystal field, establish a stable 4F3/2→4I11/2 transition with a primary emission wavelength of 1064 nm and a full width at half maximum of only 0,6 nm. This feature makes it suitable for continuous high-power laser applications. Industrial Nd:YAG lasers (e.g. IPG YLR-5000) can deliver an average output of several kilowatts and achieve beam quality with M² < 1,1 as measured by appropriate metrics. The thermal conductivity of YAG crystals is approximately 14 W/(m-K); this is considerably higher than that of glass-based materials. Owing to their isotropic thermal expansion (α ≈ 7,8×10⁻⁶ K⁻¹), thermal lensing effects at high repetition rates (>100 kHz) are minimised.
In the mid-infrared range, holmium-doped YAG (Ho:YAG) provides 2,1 μm laser emission that matches the water absorption peak (absorption coefficient α ≈ 12 cm⁻¹). Commercial devices (e.g. Coherent VersaWave) can provide pulse energies up to 5 J with controllable penetration depth. In contrast, erbium-doped YAG (Er:YAG) emits at 2,94 μm, which corresponds to the absorption peak of hydroxyl radicals; this confines thermal damage to less than 10 μm when used in enamel ablation. In passive Q-switching, chromium-doped YAG (Cr4+:YAG) is favoured because it has a high damage threshold (>500 MW/cm²) together with adjustable transmission (70–95 %), ensuring the generation of nanosecond pulses (with peak powers in the GW range) in Q-switch modules, such as those provided by EKSMA Optics.
Current technological challenges involve the control of thermal effects at high power. For example, techniques such as <111> crystal orientation dicing or YAG/Yb:YAG composite crystal designs have been developed to reduce thermally induced birefringence losses to below 0,05 λ/cm. In wavelength extension research, the ultraviolet emission (330–400 nm) of Cerium-doped YAG (Ce:YAG) has been employed for photopolymerisation of photoresists. In addition, iron-doped Zinc-Germanium-Gallium Oxide Garnet (Fe:ZnGeGaO4) is studied as a possible source of terahertz radiation in the frequency range 0,1–10 THz. Low-cost processes, such as gel-spray forming of porous YAG ceramics, reduce sintering temperatures by 200 °C and achieve optical homogeneity (Δn < 5×10⁻⁶), offering potential for large-scale applications. Future directions include the development of ultrafast laser crystals (e.g. Eu3+ doping for femtosecond pulse generation) and on-chip integration techniques, for example by heterogeneously bonding micro-nano garnet waveguides on silicon photonics chips, thereby facilitating compact and multifunctional laser systems.
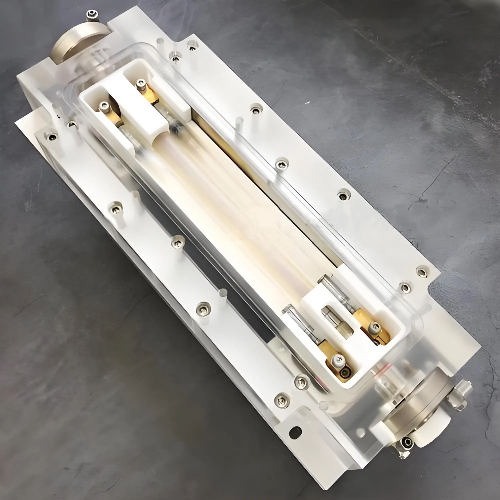
Fig. 3 YAG Laser Crystal Bar
2.3 The Importance of Comparing GGG (Gd3Ga5O12), GGAG (Gd3Ga2Al3O12) and TGG (Tb3Ga5O12)
GGG (Gd3Ga5O12), GGAG (Gd3Ga2Al3O12) and TGG (Tb3Ga5O12) all belong to the garnet family, but differences in the substitution strategies (i.e. the modulation of the rare earth ions on the A-site relative to the Al/Ga ratio on the B/C-site) lead to significant variations in their physico-chemical properties. GGG is an ideal substrate for mid-infrared lasers (e.g. Ho:GGG) and epitaxial magnetic layers (e.g. YIG) because of its wide optical transmission (0,3–6 μm) and excellent lattice matching. GGAG, by partial replacement of Ga3+ with Al3+ at the B/C sites, exhibits improved lattice rigidity and thermal conductivity (up to 9,2 W/m-K), making it suitable for heat management in high-power lasers and for scintillator applications (e.g. Ce:GGAG). TGG, with its strong magneto-optical response resulting from the 4f-electron configuration of Tb³⁺, exhibits a figure of merit (FOM) that is more than three times that of GGG and is therefore vital for optical isolators in fibre communication. Neglecting the distinction between these properties can result in technical compromises, such as the inappropriate use of GGG in high-power laser applications that may induce thermal lensing, or the wrong material selection of TGG in radiation detection where the signal-to-noise ratio is affected. The systematic comparison not only confirms the logic in correlating composition–structure–properties–application, but also provides a basic framework for designing garnet materials through targeted ion substitution. This comparative study furnishes a technical basis for the development of composite crystals (e.g. Tb–Al codoped gradient materials) and assists the industry in evaluating trade-offs between cost, performance and reliability, thereby encouraging innovation in fields such as optoelectronics, quantum technology and detection in extreme environments.
3 Comparison of Crystal Structures and Preparation Methods
3.1 Crystal Structure and Chemical Composition
GGG (Gd3Ga5O12), GGAG (Gd3Ga2Al3O12) and TGG (Tb3Ga5O12) all share the garnet framework of the cubic crystal system (space group Ia3̅ or Ia3d). However, differences in their chemical compositions cause measurable variations in the lattice parameters and ion occupancy:
1. GGG: The dodecahedral A-site is occupied by Gd3+ while the octahedral (B-site) and tetrahedral (C-site) positions are occupied by Ga3+. The lattice constant is a = 12,38 Å. This cubic structure maintains high symmetry and gives a broad transmission range (0,3–6 μm) without absorption by the high-energy band of Al3+, thus maintaining the infrared transmission required for mid-infrared laser applications.
2. GGAG: Partial substitution of Ga3+ with Al3+ improves phonon transport and increases thermal conductivity by 23%, with the lattice contracting to a = 12,12 Å. The Al–O bond length is 1,85 Å, which is shorter than the Ga–O bond length of 1,92 Å. The smaller ionic radius of Al3+ (0,39 Å compared to 0,47 Å for Ga3+) reduces lattice distortion, thereby enhancing thermal conductivity (9,2 W/m-K versus 7,5 W/m-K).
3. TGG: Tb³⁺ replaces Gd³⁺ on the A-site (ionic radius Tb³⁺ 1,04 Å vs. Gd³⁺ 1,06 Å), resulting in minimal lattice distortion (a = 12,30 Å). However, the 4f⁷ electron configuration produces strong magneto-optical effects; the field constant is approximately 3,5 times that of GGG. The interaction of the 4f⁷ electrons of Tb3+ with the crystal field significantly increases the Faraday rotation angle (−134 vs. −38 rad T⁻¹ m⁻¹).
Fig. 4 Crystal Structure of Garnet
The comparison shows that while the three crystals possess the same garnet framework, the chosen elemental substitution strategy directly governs their functional limits. Each garnet material provides specific capabilities in terms of thermal stability, optoelectronic properties and chemical flexibility. GGG is preferred for its broad infrared transmission and lattice compatibility in mid-infrared laser systems and epitaxial substrates, while GGAG—with its aluminium‐induced lattice contraction—improves thermal conductance and resistance to radiation, making it indispensable for high‐power lasers and scintillator applications. TGG utilises the strong magneto-optical response of Tb³⁺ in applications that require high performance in fibre optic communication. Despite prior successes, a systematic comparison correlating structure variations (e.g. Al/Ga ratio, Tb3+ substitution) with performance thresholds is still required. This study provides a guideline for adapting garnet crystals to the optical systems used by current research and industrial applications.
3.2 Preparation Process
The preparation processes for GGG (Gd3Ga5O12), GGAG (Gd3Ga2Al3O12) and TGG (Tb3Ga5O12) are all based on high-temperature melt growth techniques, though differences in chemical composition introduce significant variations in specific process parameters and key control factors. The comparison below focuses on three aspects: raw material processing, growth method and post-treatment procedures.
High-purity oxide precursors (such as Gd2O3, Ga2O3, Al2O3, Tb4O7 and others, with purity ≥99,99%) are essential. The Czochralski method is used in all three cases to produce single crystals by rotating the seed crystal and slowly withdrawing it from the melt. The Floating-Zone (FZ) method is sometimes used to produce high-purity crystals in order to avoid contamination from the crucible. The growth process is conducted under an inert atmosphere (argon or N2) to prevent oxidative loss of volatile components such as Gd2O3 and Tb2O3.
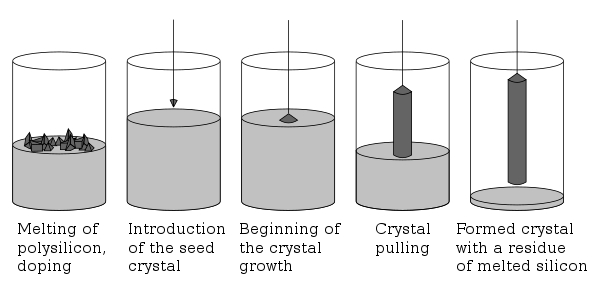
Fig. 5 Czochralski Process
Although the high-temperature melt method is common to all three materials, component characteristics (e.g. the volatility of Ga/Al/Tb, the viscosity of the melt, and oxidation tendencies) require differentiated process control. In the growth of GGG, volatilisation of Gd2O3 leads to melt non-stoichiometry, which necessitates real-time monitoring and the addition of excess Ga2O3 (~1 wt.-%) to compensate for a volatilisation rate of approximately 3% per hour at 1800 °C. To minimise losses from thermal convection, a double-layered crucible (inner layer of Ir and outer layer of Mo) may be used. In GGAG growth, differences in melt viscosity between Al2O3 and Gd2O3 may lead to component segregation (with Al enrichment at the edges); this is mitigated by employing an ultrasonic-assisted melt mixing at 20 kHz coupled with a slow rotation (<15 rpm). During TGG growth, maintaining interface stability is critical due to the high melting point of Tb2O3 (~2200 °C). The process requires higher growth temperatures and is vulnerable to thermally induced stress fractures; such microcracks are removed by applying a heating gradient of 5 °C/min combined with hot isostatic pressing (HIP) at 1500 °C/100 MPa in argon.
Table 1: Comparison of Growth Process Control Parameters
|
Process Parameter |
GGG |
GGAG |
TGG |
|
Control of Melt Volatilisation |
Inhibition of Ga2O3 volatilisation: An excess of Ga2O3 (~1 wt.-%) is added to compensate, with a loss rate of approximately 3% per hour at 1800 °C. |
Regulation of Al2O3 doping: The melt viscosity is high (η ≈ 30 mPa·s at 1800 °C) and rotation rates (10–20 rpm) must be optimised for homogeneity. |
Stability of Tb2Oₓ: Tb3+ is prone to oxidation to Tb4+, thereby requiring strict control of the oxygen partial pressure (PO2 ≈ 10⁻⁵ atm). |
|
Growth Temperature |
1780–1820 °C |
1750–1800 °C (Al lowers the melting point) |
1850–1900 °C (Tb increases the melting point) |
|
Interface Stability |
Flat interface growth (ΔT < 5 °C) |
Required to suppress Al segregation (ΔAl < 2%) |
High melt temperature leads to a volatile solid–liquid interface (ΔT < 3 °C required) |
|
Post-Treatment Process |
Annealing: 1200 °C in Ar for 24 h to remove Ga vacancies |
Oxygen vacancy repair: 1300 °C in O₂ for 12 h to improve Ce³⁺ luminescence efficiency |
Magnetic domain optimisation: 1400 °C annealing in a H₂/Ar mixed atmosphere to improve magneto-optical uniformity |
Table 2: Effect of Process Comparison on Applications
|
Material |
Process Challenges |
Impact on Performance |
Typical Optimisation Result |
|
GGG |
Control of Ga2O3 volatilisation |
Optical homogeneity (Δn < 1×10⁻⁵) |
Φ150 mm single crystal (substrate for optical communication) |
|
GGAG |
Uniform distribution of aluminium |
Scintillator light output uniformity (±3%) |
Ce:GGAG ceramic (light yield of 55 000 photons/MeV) |
|
TGG |
High-temperature interface stability |
Magneto-optical uniformity (Δθ < 0,01°/mm) |
Φ100 mm single crystal (5G isolator) |
4 Comparative Analysis of the Physical and Chemical Properties
The differences in the physico-chemical properties of GGG, GGAG and TGG arise from the specific modulation of their elemental compositions and crystal structures. These differences directly affect their suitability for various applications. The analysis below compares the thermal, optical and mechanical/radiative properties:
4.1 Thermal Properties
Thermal conductivity: GGAG has a thermal conductivity of 9,2 W/(m-K), which is higher than that of GGG (7,5 W/(m-K)) and TGG (6,8 W/(m-K)). This makes GGAG suitable for high heat dissipation in laser systems.
Thermal expansion coefficient: TGG exhibits a slightly higher thermal expansion coefficient (8,5×10⁻⁶ K⁻¹) owing to magnetostrictive effects of Tb3+ (magnetocrystalline coupling coefficient λ₁₁ ≈ −1,2×10⁻⁶). In contrast, GGAG (7,3×10⁻⁶ K⁻¹) and GGG (7,9×10⁻⁶ K⁻¹) show more isotropic thermal expansion, making them suitable for optical components used in high-temperature environments.
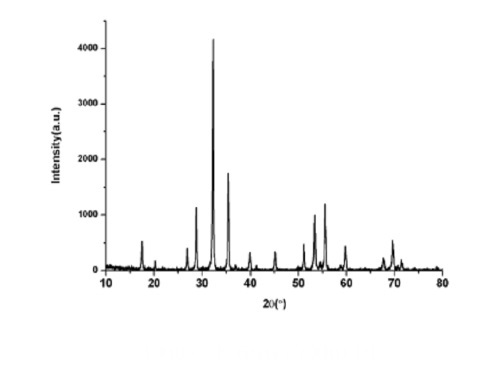
Fig. 6 XRD Pattern of GGG at 1000 °C
4.2 Optical Properties
GGG exhibits a broad transmission range covering the mid-infrared (3–5 μm), which is suitable for CO₂ laser transmission (e.g. 10,6 μm window material).
GGAG enhances blue light transmission with a band from 400 to 500 nm showing a transmission of over 85% (compared to 75% for GGG), making it appropriate for applications requiring high scintillator light output.
TGG displays a strong magneto-optical response. Its field constant is −134 rad T⁻¹ m⁻¹ at 632 nm as opposed to −38 rad T⁻¹ m⁻¹ for GGG, permitting a reduction in the dimensions of optical isolators.
Table 3: Comparison of the Optical Properties of GGG, GGAG and TGG
|
Parameter |
GGG |
GGAG |
TGG |
|
Transmission Range |
0,3–6 μm |
0,25–5 μm (blue light enhancement) |
0,4–5 μm |
|
Field Constant |
−38 rad T⁻¹ m⁻¹ at 632 nm |
−45 rad T⁻¹ m⁻¹ at 632 nm |
−134 rad T⁻¹ m⁻¹ at 632 nm |
|
Absorption Coefficient @ 1 μm |
0,05 cm⁻¹ |
0,08 cm⁻¹ |
0,12 cm⁻¹ |
4.3 Mechanical and Radiative Properties
TGG is susceptible to surface microcracks owing to lattice distortions associated with Tb3+; this necessitates process optimisation, for example through chemical-mechanical polishing.
Radiation tolerance: Under a γ-ray exposure of 10⁶ Gy, GGG exhibits a light yield reduction of ≈15%, whereas GGAG shows a reduction of less than 5% due to the inhibiting effect of Al³⁺ on oxygen vacancies (oxygen vacancy concentration < 10¹⁶ cm⁻³). The Ce:GGAG scintillator retains more than 90% of its original light output after a dose of 100 kGy, which is markedly better than that of conventional BGO crystals.
Table 4: Comprehensive Performance Comparison
|
Parameter |
GGG |
GGAG |
TGG |
Core Application Impact |
|
Thermal Conductivity |
7,5 W/(m-K) |
9,2 W/(m-K) |
6,8 W/(m-K) |
GGAG is suited to high heat dissipation conditions |
|
Field Constant |
−38 rad T⁻¹ m⁻¹ |
−45 rad T⁻¹ m⁻¹ |
−134 rad T⁻¹ m⁻¹ |
TGG is preferred for miniaturised magneto-optical isolators |
|
Mohs Hardness |
7,8 |
8,2 |
7,5 |
GGAG is suitable for precise optical machining |
|
Radiation Stability |
ΔLY ≈ 15% @ 10⁶ Gy |
ΔLY < 5% @ 10⁶ Gy |
ΔLY ≈ 20% @ 10⁶ Gy |
GGAG is appropriate for high-dose environmental detection |
GGG, GGAG and TGG are each suited to different applications due to marked differences in their core properties. GGG is selected for mid-infrared laser systems (e.g. Ho:GGG lasers) and thin-film epitaxial substrates (for YIG growth); GGAG, with improved thermal conductivity (9,2 W/(m-K)) and radiation stability (optical output reduction < 5% upon 10⁶ Gy), is well-suited to heat dissipation modules in high-power lasers and scintillator applications; TGG, on account of its high magneto-optical performance (Verdet constant: −134 rad T⁻¹ m⁻¹) and high damage threshold (above 500 MW/cm²), is integral to optical isolators in fibre communication as employed in systems such as 5G photonic switches. The complementary properties of these materials underscore the importance of a cross-material approach for addressing multiple technological scenarios in integrated laser-magneto-optical systems.
5 Application Scenarios and Case Studies
5.1 Core Applications of GGG
1. Substrate materials for mid-infrared lasers
Beneficial bandwidth: GGG exhibits a considerably broader transmission range (0,3–6 μm) than YAG (0,4–5 μm). This is particularly significant in the atmospheric window band of 3–5 μm. This property is critical for gas detection and for use in infrared countermeasure systems.
Typical doping system:
Ho:GGG emits laser light at 2,1 μm with a water absorption coefficient (α ≈ 12 cm⁻¹), which is calibrated for procedures such as prostate ablation (pulse energy 5 J as used in devices from Boston Scientific);
Er:GGG provides 2,8 μm laser output that is applied in dentin ablation (pulse energy 300 mJ at a repetition rate of 10 Hz), with a thermal damage layer thickness below 20 μm.
Thermal management: Despite a thermal conductivity of 7,5 W/(m-K) – slightly lower than that of GGAG – the isotropic thermal expansion (α ≈ 7,9×10⁻⁶ K⁻¹) reduces thermally induced birefringence. This ensures a high beam quality (M² < 1,2).
Fig. 7 Substrate Materials for Infrared Lasers
2. Magnetic thin-film epitaxial substrates
Lattice compatibility: The lattice mismatch between GGG and Yttrium Iron Garnet (Y3Fe5O12, YIG) is only 0,03% (GGG with a cell parameter of 12,38 Å versus 12,376 Å for YIG), which provides a basis for low-defect epitaxy.
Applications include:
– Deposition of bismuth-doped YIG (Bi:YIG) on GGG substrates, achieving a Faraday rotation of up to 0,041°/μm at 1550 nm with an insertion loss of less than 0,2 dB;
– Construction of spin-wave devices using YIG/GGG heterojunctions operating at frequencies between 1 and 20 GHz.
Industrial advantages include a substrate cost reduction of approximately 40% when compared with a single crystal of equivalent size, and the ability to polish and reuse the substrate for over 50 epitaxial cycles.
3. Optical windows for extreme environments
These windows benefit from high temperature and thermal shock resistance. GGG exhibits less than a 5% reduction in infrared transmission at 1200 °C (compared to over 15% damping in YAG), making it suitable for monitoring combustion chambers in aero-engine applications. GGG also shows better resistance to particle irradiation, with a bulk absorption change Δα of less than 0,01 cm⁻¹ upon proton injection at a fluence of 10¹⁴ protons/cm², which is an improvement over sapphire (which shows Δα ≈ 0,05 cm⁻¹). This makes it suitable for diagnostic windows in nuclear fusion facilities.
5.2 The Indispensability of TGG
1. Magneto-optical isolators for fibre optic communication
Miniaturised design: The high field constant of TGG reduces the isolator length to one-third of that required for GGG. For instance, a 1550-nm device requires only 5 mm to achieve 40 dB isolation. This reduction supports the compact design of optical 5G modules (dimensions less than 10×10×5 mm³).
High power tolerance: Under continuous laser operation of 100 W (with a core diameter of 10 μm), the temperature rise in the TGG isolator remains under 5 °C (compared to more than 15 °C for GGG), thereby ensuring stable optical connections in data centres with an insertion loss of less than 0,3 dB.

Fig. 8 Magneto-optical Isolators for Fibre Optic Communications
2. High-power laser systems
Pulsed laser modulation: TGG functions as the Faraday rotator to shape nanosecond pulses (with pulse widths between 10 and 50 ns and a repetition rate of 100 kHz) in a fibre laser system rated at 10 kW, with peak power densities exceeding 1 GW/cm².
Thermal management strategy: A TGG/AlN composite structure is employed for efficient heat dissipation. This strategy achieves an interfacial thermal resistance of less than 1×10⁻⁵ m²·K/W, thereby reducing thermally induced depolarisation losses to below 0,05 λ/cm.
3. Carrier for quantum technology
In TGG, electron spins (in the ground state 7F6) of Tb3+ have coherence times (T₂) of up to 15 μs at 4 K. This makes them suitable for solid-state quantum memory applications, with single-photon fidelities exceeding 99%. Additionally, TGG crystals are used to generate magnetic field gradients (>50 G/cm/mm) suitable for integration with cold atom chips.
5.3 The Emerging Direction for GGAG
1. Heat dissipation and gain media for high-power lasers
GGAG’s thermal conductivity of 9,2 W/(m-K) is 23% higher than that of GGG. This improvement permits its use in the thermal management of fibre lasers rated at 10 kW, achieving a temperature rise reduction of approximately 40%. For example, systems such as the YLS-10000 from IPG Photonics utilise GGAG ceramic heat sinks.
UV pump compatibility: Aluminium doping shifts the absorption edge to 250 nm (compared to 300 nm for GGG), which is beneficial for pumping Nd:YAG lasers at the third harmonic (355 nm) in conjunction with Ce activation. This results in a fluorescence conversion with a light output exceeding 200 lm/W.

Fig. 9 Heat Dissipation and Gain Media for High-Power Lasers
2. Radiation detection and imaging
Ce³⁺-activated GGAG scintillators can deliver up to 55 000 photons/MeV with decay times of 60 ns, making them suitable for time-of-flight PET detectors with time resolutions of less than 300 ps (as employed in systems such as the Siemens Biograph Vision).
High temperature and radiation tolerance: At 150 °C, GGAG retains over 90% of its optical output, compared with only 50% for BGO crystals. This makes it appropriate for neutron monitoring in nuclear reactor environments.
3. Transparent ceramics and photonic devices
Large-scale production: Transparent GGAG ceramics with diameters up to Φ150 mm (with transmission above 80% at 600 nm) can be produced by nanopowder sintering using the HPHIP process, resulting in a cost reduction of approximately 60% compared with single crystals. These ceramics are being used in beam smoothing devices for laser fusion applications (such as those in the NIF upgrade project).
Nonlinear optics: An optical parametric oscillator (OPO) for the mid-infrared, tuned over the range 3–5 μm, has been developed utilising the high damage threshold (greater than 1 GW/cm²) and broad transmission range of GGAG.
6 Directions and Perspectives for Future Challenges
Future development of GGG will focus on the growth of larger crystals and expansion of functional capabilities. Growth breakthroughs to produce Φ200 mm single crystals are required to meet the demand for 8‑inch wafer epitaxy (as used in ASML photolithography laser modules). This improvement must be accompanied by a reduction in the concentration of oxygen vacancies through Eu³⁺ codoping to below 10¹⁵ cm⁻³, thereby enhancing ultraviolet–visible transmission (targeting greater than 80% transmission at 400 nm). Advances in GGG-based gradient refractive index (GRIN) lenses, integrating laser emission and beam shaping for compact laser systems (with beam quality M² < 1,05), are also under investigation for applications in diffraction-limited modulation in optical space communication.
Research on TGG will prioritise performance optimisation and sustainable practices. Efforts include reducing lattice distortion (Δa < 0,01 Å) and improving optical homogeneity (Δn < 1×10⁻⁶) by means of La³⁺ codoping and designing a Ce³⁺/Tb³⁺ energy transfer system to increase the magneto-optical effect in the UV–visible range (with a target improvement of 20% in the field constant at 400 nm). In heterogeneous integration, TGG/SiN hybrid components are being developed for photonic chips (with edge coupling losses less than 0,5 dB) for modulating quantum light sources, as well as for terahertz switches using TGG–graphene heterojunctions (exhibiting interpolation losses between 0,1 and 3 THz of less than 2 dB). For environmentally sustainable fabrication, a recycling rate exceeding 95% for terbium is being targeted, thereby reducing dependence on rare earth elements.
Innovations in GGAG concentrate on defect modulation and adaptation to extreme environmental conditions. The energy resolution of Ce:GGAG scintillators is being improved to below 5% at 662 keV by compensating the Al3+ charge imbalance through Mg²⁺ codoping; additionally, gradient designs with an aluminium content ranging from 20% to 80% are implemented to mitigate thermal stress and enhance ceramic crack resistance by 50%. In the photonic integration domain, a garnet-based photonic crystal fibre (PCF) is being developed to achieve high-power laser transmission with losses lower than 0,1 dB/m at 1 μm, and a micro-nano waveguide quantum dot coupling system is being designed to achieve single-photon emission purities exceeding 99%. For extreme environment applications, radiation sensors for space with operating temperatures between −200 and +300 °C and optical monitoring windows for fusion reactors with resistance to neutron fluences greater than 10²⁰ n/cm² are under development to support projects such as ITER.
7 Conclusion
The comparative analysis of GGG, GGAG and TGG garnet crystals demonstrates the significant effects that targeted elemental substitutions have on their structural, thermomechanical and photonic properties. GGG’s broad infrared transmission and lattice compatibility secure its role in mid-infrared laser systems and epitaxial substrates. GGAG benefits from aluminium-mediated lattice contraction, which enhances thermal conductivity (9,2 W/(m-K)) and radiation resistance making it indispensable for high-power laser heat dissipation modules and scintillator detectors. TGG, with its high magneto-optical performance (Verdet constant of −134 rad T⁻¹ m⁻¹), is used in optical isolators for fibre communication and in applications related to quantum technologies. The differing yet complementary functionalities of these materials are a result of tuning the rare earth content on the A-site and controlling the Ga/Al ratio on the B/C-site. The advances in defect management (e.g. reducing oxygen vacancies in GGAG), the design of hybrid crystals (e.g. Tb/Al codoped gradients) and scalable synthesis techniques are required to overcome cost and size limitations. By linking crystal engineering with specific photonic requirements, this study provides a framework for optimising garnet-based systems in integrated optoelectronics, extreme environment sensing and future quantum devices.
Further reading:
Innovations in Optics: The Role of GGG-, SGGG- and NGG-Garnet Boules
GGG vs. SGGG Crystal Substrates: Which is the Better Choice for Your Technical Requirements?

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us



 Chin Trento
Chin Trento



