List Of Superconductors And How They Work
Introduction
Superconductivity is a phenomenon in physics observed in certain materials when they are cooled below a specific critical temperature. Under these conditions, the material exhibits zero electrical resistance and expels magnetic fields. This effect is employed in various applications, for example in medical imaging, energy storage and transportation. In the following, we explain the operating mechanism of superconductors using ten examples of superconducting materials.
How Superconductors Function
Superconductivity occurs when the electrons in a material form what are known as Cooper pairs. These pairs traverse the material without scattering, thereby preventing energy dissipation. In conventional conductors, for instance copper or aluminium, electrons collide with atoms and cause resistance, which results in energy loss. Once the material is cooled below its critical temperature, current flows without dissipation.
At the quantum level, the BCS theory (Bardeen, Cooper and Schrieffer) explains superconductivity. This theory describes how interactions between electrons and lattice vibrations result in the formation of Cooper pairs. Consequently, the material is capable of conducting electricity without energy loss.
Superconducting Properties
Superconductors exhibit a range of properties that set them apart from other materials:
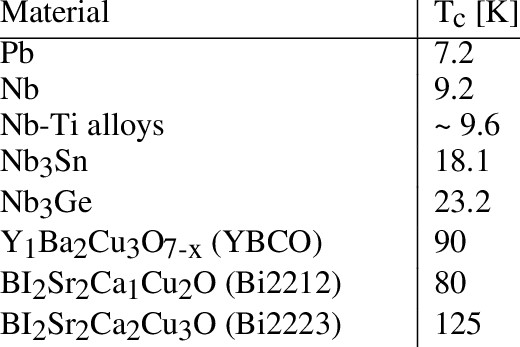
Figure 1 – Critical temperature of superconductors [1]
- No electrical resistance: Superconductors allow current to flow with zero resistance, thereby avoiding energy losses during transmission.
- Meissner effect: Superconductors expel magnetic fields from their interior upon entering the superconducting state. This phenomenon is utilised in applications such as magnetic levitation.
- Critical Temperature (Tc): Each superconductor has a specific temperature below which it exhibits superconductivity. For instance, some high-temperature superconductors have a critical temperature above the boiling point of liquid nitrogen (−196 °C).
- Quantum levitation state: Superconductors can levitate above magnets as a result of the interaction between the magnetic fields expelled by the superconductor and those produced by a magnet. This principle is applied in technologies including maglev trains.
- High current carrying capacity: Superconductors can conduct considerably higher electrical currents than conventional conductors, making them suitable for high-energy applications such as particle accelerators.
Ten Examples of Superconductors
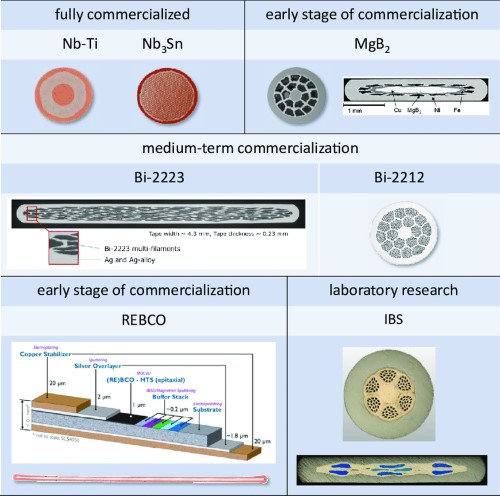 [2]
[2]
- Niobium (Nb) Niobium is one of the most frequently used superconductors. It exhibits a critical temperature of 9.25 K and is applied in devices such as MRI scanners and particle accelerators.
- Yttrium-Barium-Copper Oxide (YBCO) YBCO is a high-temperature superconductor with a critical temperature of approximately 93 K. It is used in the energy sector, for instance in superconducting cables and magnetic shielding.
- Magnesium Diboride (MgB2) Magnesium diboride, with a critical temperature of 39 K, is a cost-effective superconductor. It finds applications in electronics, energy storage and MRI technology.
- Lead (Pb) Lead was among the first materials to exhibit superconductivity. Its critical temperature is 7.2 K, and it is used in scientific experiments and applications that operate at low temperatures.
- Bismuth-Strontium-Calcium-Copper Oxide (BSCCO) BSCCO is another high-temperature superconductor with a critical temperature of about 108 K. It is implemented in power cables, magnets and various electrical devices.
- Iron-based Superconductors Discovered in 2008, this class is noted for its relatively high critical temperatures and potential in electronic and energy applications.
- Tungsten (W) Tungsten is a dense material that becomes superconducting at very low temperatures, making it suitable for niche applications, including high-field magnets.
- Vanadium-Gallium (V3Ga) Vanadium-gallium has a critical temperature of 13.8 K. It is employed in applications that require both superconductivity and high magnetic fields.
- Copper Oxide (CuO) Copper oxide is an example of a high-temperature superconductor operating above 77 K (the boiling point of liquid nitrogen). Modern electrical and electronic devices utilise this material.
- Lanthanum-Strontium-Copper Oxide (LSCO) LSCO belongs to the high-temperature superconductor class and is used in research and electronics, particularly in devices where minimal energy loss is required.
List of Superconductors
Below is an overview table with common examples of superconductors. For further details and examples, please refer to Stanford Advanced Materials (SAM).
|
Substance |
Class |
Tc (K) |
Hc (T) |
Type |
|
Al |
Element |
1.20 |
0.01 |
I |
|
Bi |
Element |
5.3×10-⁴ |
5.2×10-⁶ |
I |
|
Cd |
Element |
0.52 |
0.0028 |
I |
|
Diamond:B |
Element |
11.4 |
4 |
II |
|
Ga |
Element |
1.083 |
0.0058 |
I |
|
Element |
0.165 |
- |
I |
|
|
α-Hg |
Element |
4.15 |
0.04 |
I |
|
β-Hg |
Element |
3.95 |
0.04 |
I |
|
In |
Element |
3.4 |
0.03 |
I |
|
Ir |
Element |
0.14 |
0.0016 |
I |
|
α-La |
Element |
4.9 |
- |
I |
|
β-La |
Element |
6.3 |
- |
I |
|
Li |
Element |
4×10-⁴ |
- |
I |
|
Mo |
Element |
0.92 |
0.0096 |
I |
|
Element |
9.26 |
0.82 |
II |
|
|
Os |
Element |
0.65 |
0.007 |
I |
|
Pa |
Element |
1.4 |
- |
I |
|
Pb |
Element |
7.19 |
0.08 |
I |
|
Element |
2.4 |
0.03 |
I |
|
|
Rh |
Element |
3.25×10-⁴ |
4.9×10-⁶ |
I |
|
Ru |
Element |
0.49 |
0.005 |
I |
|
Si:B |
Element |
0.4 |
0.4 |
II |
|
Sn |
Element |
3.72 |
0.03 |
I |
|
Element |
4.48 |
0.09 |
I |
|
|
Tc |
Element |
7.46-11.2 |
0.04 |
II |
|
α-Th |
Element |
1.37 |
0.013 |
I |
|
Ti |
Element |
0.39 |
0.01 |
I |
|
Tl |
Element |
2.39 |
0.02 |
I |
|
α-U |
Element |
0.68 |
- |
I |
|
β-U |
Element |
1.8 |
- |
I |
|
V |
Element |
5.03 |
1 |
II |
|
α-W |
Element |
0.015 |
0.00012 |
I |
|
β-W |
Element |
1-4 |
- |
I |
|
Yb |
Element |
1.4 (>86 GPa) |
- |
keine |
|
Zn |
Element |
0.855 |
0.005 |
I |
|
Element |
0.55 |
0.014 |
I |
|
|
Ba8Si46 |
Clathrate |
8.07 |
0.008 |
II |
|
CaH6 |
Clathrate |
215 (172 GPa) |
- |
II |
|
C6Ca |
Compound |
11.5 |
0.95 |
II |
|
C6Li3Ca2 |
Compound |
11.15 |
- |
II |
|
C8K |
Compound |
0.14 |
- |
II |
|
C8KHg |
Compound |
1.4 |
- |
II |
|
C6K |
Compound |
1.5 |
- |
II |
|
C3K |
Compound |
3.0 |
- |
II |
|
C3Li |
Compound |
<0.35 |
- |
II |
|
C2Li |
Compound |
1.9 |
- |
II |
|
C3Na |
Compound |
2.3-3.8 |
- |
II |
|
C2Na |
Compound |
5.0 |
- |
II |
|
C8Rb |
Compound |
0.025 |
- |
II |
|
C6Sr |
Compound |
1.65 |
- |
II |
|
C6Yb |
Compound |
6.5 |
- |
II |
|
Sr2RuO4 |
Compound |
0.93 |
- |
II |
|
C60Cs2Rb |
Compound |
33 |
- |
II |
|
C60K3 |
Compound |
19.8 |
0.013 |
II |
|
C60RbX |
Compound |
28 |
- |
II |
|
C60Cs3 |
Compound |
38 |
- |
II |
|
FeB4 |
Compound |
2.9 |
- |
II |
|
InN |
Compound |
3 |
- |
II |
|
In2O3 |
Compound |
3.3 |
~3 |
II |
|
Compound |
0.45 |
- |
II |
|
|
MgB2 |
Compound |
39 |
74 |
II |
|
Nb3Al |
Compound |
18 |
- |
II |
|
NbC1-xNx |
Compound |
17.8 |
12 |
II |
|
Nb3Ge |
Compound |
23.2 |
37 |
II |
|
NbO |
Compound |
1.38 |
- |
II |
|
NbN |
Compound |
16 |
- |
II |
|
Nb3Sn |
Compound |
18.3 |
30 |
II |
|
NbTi |
Compound |
10 |
15 |
II |
|
SiC:B |
Compound |
1.4 |
0.008 |
I |
|
SiC:Al |
Compound |
1.5 |
0.04 |
II |
|
TiN |
Compound |
5.6 |
5 |
I |
|
V3Si |
Compound |
17 |
- |
II |
|
YB6 |
Compound |
8.4 |
- |
II |
|
ZrN |
Compound |
10 |
- |
I |
|
ZrB12 |
Compound |
6.0 |
- |
II |
|
Ute2 |
Compound |
2.0 |
- |
- |
[3]
Conclusion
With zero electrical resistance and distinct magnetic properties, superconductors are used in technical fields ranging from medical imaging to transportation. Further research is expected to yield new materials with higher critical temperatures, thereby increasing application possibilities.
References:
[1] Lebrun, P., Tavian, L., Vandoni, G. and Wagner, U. (2002). Cryogenics for Particle Accelerators and Detectors.
[2] Yao, C. and Ma, Y. (2021). Superconducting Materials: Challenges and Opportunities for Large-scale Applications. iScience, 24, 102541. https://doi.org/10.1016/j.isci.2021.102541.
[3] List of Superconductors. (16/08/2024). In Wikipedia. https://en.wikipedia.org/wiki/List_of_superconductors

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us





 Chin Trento
Chin Trento



