Why Can Rare-earth Elements Be Used For Dental Coloring?
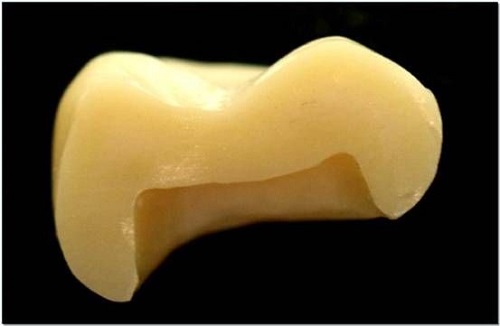
Yttrium-stabilised Zirconium Dioxide is one of the most commonly used ceramic materials in daily life. It exhibits high mechanical performance at room temperature, is biocompatible and has low thermal conductivity. Consequently, it has become an appropriate ceramic material for dental restoration. If it is not treated with a dye, the material shows a chalky colour that does not meet the clinical standards for dental prostheses colour matching.
To replicate the appearance of natural teeth, the material is dyed during clinical application. Rare earth elements possess distinct optical properties and stable colours and are frequently used in ceramics. When colouring dental materials, the hue range corresponding to natural teeth and the elemental properties limitations must be considered. At present, only a few rare earth elements are used for colouring dental materials, primarily Ce, Pr, Er, Nd, etc.
Colouring Method for Rare Earth Dyes
Immersion dyeing
To achieve the colour reproduction of natural teeth, immersion dyeing is often employed in clinical practice, that is, the dyeing of zirconia blanks following presintering. The dyeing method is simple to operate; however, it may lead to uneven colour distribution because of operational factors. At present, many dye solutions available on the market are primarily manufactured using rare earth elements.
Powder mixing
In powder mixing, the dye is combined with the zirconium dioxide starting powder prior to high-temperature sintering. At elevated temperatures, the colouring ions diffuse into the ZrO2 lattice as a solid solution and impart colour. This method minimises uneven colour distribution arising from factors such as the thickness of the preheated body, microstructure and the clinical dyeing process, thereby exerting only a limited effect on the material’s brightness. However, the properties of the sintered materials are affected by the modification of the zirconium dioxide powder when the dye is doped.
Effects of Colouring with Rare Earth Metals on Material Properties
There are two key requirements for dental ceramics in clinical practice: mechanical performance and biocompatibility. Zirconium dioxide ceramics satisfy both criteria; however, the crystal phase characteristics and the material density are influenced by the dye doping. Therefore, it is necessary to minimise the impact on material properties while enhancing their appearance with rare earth dyes.
Changes in mechanical properties
Studies have shown that the maximum bending strength of human premolars can reach 700 N or higher. Consequently, the bending strength of ceramic materials used in clinical prostheses should exceed 800 MPa. According to the presented study, the addition of trace elements may affect the mechanical properties of zirconium dioxide. Provided that the quantity of the doped oxide is properly controlled, the clinical performance is not compromised; occasionally, certain dye treatments improve the mechanical properties of the material.
Study on the cytotoxicity of dyed materials
Before clinical application, medical biomaterials must undergo scientific biosafety tests, with cytotoxicity being one of the most important indices of biosafety. The rare earth elements used for the colouring of full-ceramic dental materials primarily belong to the group of light rare earth elements. Although light rare earth elements may exhibit some toxicity, their low concentrations have significant anticancer and antimutagenic effects, and their addition as a dye is minimal.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us


 Chin Trento
Chin Trento


