Discovery Of The Elements
Introduction
The discovery of elements is underpinned by systematic historical research. Researchers have examined the fundamental substances that constitute matter for many centuries. This article outlines the development of our understanding of elements from ancient times to modern research, and it details key experimental findings and the scientists responsible for these advancements.
Ancient Insights
Early documented research on elements dates back to ancient civilisations. Greek philosophers such as Empedocles proposed that four primary elements exist: earth, water, air and fire. This framework influenced scientific investigations for many centuries.
Alchemical Investigations
During the Middle Ages and Renaissance, alchemists conducted experiments to convert common metals into recognised noble metals. They also aimed to isolate the philosopher’s stone and identify life-sustaining substances. Their work established a basis for systematic chemical experimentation, which later enabled the discovery of new elements.
The Age of Enlightenment
The eighteenth century marked a period of precise experimental work. Antoine Lavoisier conducted experiments that refuted the classical theory of elements. He established the Law of Conservation of Mass and defined chemical elements as substances that cannot be decomposed through chemical reactions.
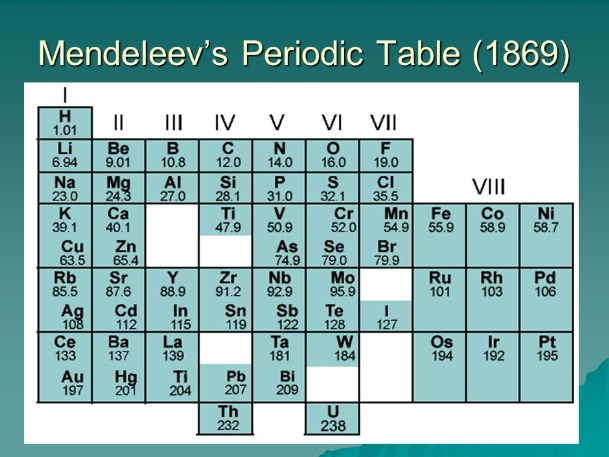
The Periodic Table
In the nineteenth century, researchers achieved significant progress in element discovery. Dmitri Mendelejew organised elements by atomic weight and chemical properties. His table allowed predictions of unknown elements. His forecasts were confirmed when elements such as gallium and germanium were identified.
Synthesis of Synthetic Elements
As the periodic table expanded, certain elements were not found naturally and were instead produced in laboratory conditions. Glenn T. Seaborg led efforts to synthesise transuranic elements such as americium and curium. His experimental work extended the periodic table beyond uranium.
The Emergence of Modern Chemistry
The twentieth century delivered further advances. Nuclear physics and particle accelerators enabled the creation and identification of synthetic elements. Researchers, including Seaborg, synthesised several transuranic elements, thereby extending the known range of chemical elements.
Modern laboratories continue systematic research using accelerator facilities and controlled nuclear reactions. Elements with atomic numbers 113 (Nihonium, Nh), 114 (Flerovium, Fl) and 118 (Oganesson, Og) have been produced, with experimental evidence supporting their existence in the twenty-first century.
The Standard Model of Particle Physics
In particle physics, investigations also address subatomic components. The identification of quarks and their participation in forming protons, neutrons and atomic nuclei has refined our understanding of matter’s composition.
Conclusion
The discovery of elements represents a systematic effort by researchers over many centuries. From early Greek theories to modern laboratory experiments, each study has contributed measurable data to the field. The periodic table continues to serve as a fundamental framework as new elements are produced under controlled conditions. This research remains a central aspect of scientific investigation in both chemistry and physics.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Chin Trento
Chin Trento



