How To Prevent Catalyst Deactivation?
Introduction
Catalysts are essential in many industrial processes as they enable chemical reactions to occur more efficiently and at lower temperatures or pressures. However, catalysts lose activity over time, resulting in reduced efficiency and increased costs. This article discusses methods to prevent catalyst deactivation. We hope it enhances your understanding of maintaining various catalysts.
How can catalyst deactivation be prevented?
--Poisoning
The primary cause of catalyst deactivation is poisoning. This term refers to the reversible or irreversible chemical deactivation of a catalyst. It results in a loss of catalytic activity, stability and selectivity and may lead to technical issues and financial losses in industrial processes. Figure 1 shows the sulphur poisoning of nickel catalysts by H2S with and without additional oxygen.
To prevent catalyst poisoning, the catalyst may be pre-treated or removed.
- If the poisoning is reversible, the catalyst can be reused.
- If it is irreversible, the catalyst must be disposed of, an operation that incurs high energy and cost. The catalyst may be pre-treated by using ZnO and other protective agents to mitigate sulphur poisoning.
- Deactivated catalysts should be removed if complete elimination of the toxins proves very difficult.
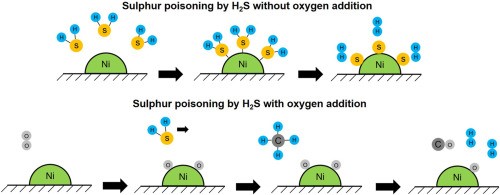
[1]
Figure 1. Sulphur Poisoning
-Sintering
Sintering is another common cause of catalyst deactivation. It results from thermal degradation and reduces both the catalytic surface area and the support area. The catalytic phase converts into non-catalytic phases, thereby obstructing the intended chemical reactions.
Please ensure the materials and environment are appropriate to prevent sintering.
- Alkali metals accelerate sintering, whereas oxides of Ba, Ca or Sr reduce the sintering rate. Porous materials show a lower sintering rate.
- Steam and chlorine accelerate sintering. In addition, humid atmospheres, overheating and surface losses expedite structural changes in oxide supports.
-Coking
Coking accounts for approximately 20 % of catalyst deactivation and is typically linked with clogging. Carbonaceous and other materials accumulate in the catalyst pores, reducing pore size and preventing reactant molecules from diffusing into them.
Normally, these carbon deposits can be removed by gasification with either steam or hydrogen, thereby producing CH4, CO or COx. Consequently, deactivation by coking is a reversible process. Figure 2 presents a schematic representation of coke deposition on unmodified and metal-modified HZSM-5 catalysts.
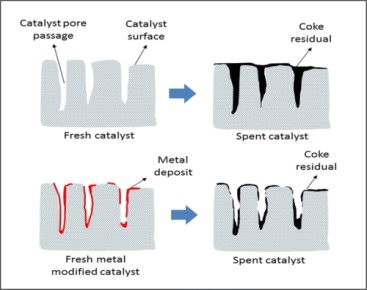
[2]
Figure 2. Coke Deposition
-Others
Several additional approaches may be applied to prevent catalyst deactivation.
- Selection of the appropriate catalyst
Choosing the correct catalyst for a given application is crucial to preventing deactivation. Different catalysts exhibit varying stability and resistance to deactivation given the process conditions. The design of the catalyst is relevant. Therefore, adjustments to the surface, pore and pellet sizes may prevent catalyst poisoning.
- Keep the catalyst clean
A major reason for catalyst deactivation is the buildup of impurities on its surface. These impurities may originate from the feedstock or the environment. Regular system flushing or feedstock filtration is recommended.
- Avoid high temperatures
Catalysts may react adversely to elevated temperatures, thereby risking deactivation. It is essential to ensure that the catalyst is not exposed to temperatures beyond its safe operating range. Monitoring system temperature and adjusting the process accordingly is advised.
- Monitor catalyst activity
Tracking catalyst activity can help detect changes in performance. Regular measurements of reaction rate or periodic catalyst tests may be conducted. Early detection of issues allows for timely corrective actions, thereby preventing deactivation.
Conclusion
In summary, follow the steps above to avoid poisoning, sintering and coking, which are the principal causes of catalyst deactivation. Additionally, the operating conditions, as well as the selection, use and maintenance of the catalyst, should be carefully controlled. This practice prolongs catalyst lifetime, thereby improving efficiency and reducing costs in industrial processes.
Stanford Advanced Materials (SAM) supplies various types of noble metal catalysts at competitive prices. Other noble metal products, such as noble metal crucibles and noble metal wires, are also available. Please visit our website for further details.
Reference:
[1] Philipp Wachter, Christian Gaber, Juraj Raic, Martin Demuth, Christoph Hochenauer, (2021). Experimentelle Untersuchung der H2S- und SO2-Schwefelvergiftung und Regeneration eines handelsüblichen Ni-Katalysators bei der Methan-Trireformierung [Photograph]. https://www.sciencedirect.com/science/article/abs/pii/S0360319920340921
[2] Balasundram, Vekes & Ibrahim, Norazana & Kasmani, Rafiziana & Isha, Ruzinah & Abd Hamid, Mohd Kamaruddin & Hasbullah, Hasrinah. (2022). Katalytische Veredelung von aus Biomasse gewonnenem Pyrolyse-Dampf über metallmodifiziertem HZSM-5 zu BTX: A comprehensive review [Photograph]. https://www.researchgate.net/publication/343461067_Catalytic_upgrading_of_biomass-derived_pyrolysis_vapour_over_metal-modified_HZSM-5_into_BTX_a_comprehensive_review

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators





 Chin Trento
Chin Trento



