Lithium Tantalate Vs. Lithium Niobate Wafers: A Comprehensive Comparison For Tech Enthusiasts
1 Introduction
Lithium niobate (LiNbO3, LN) and Lithium tantalate (LiTaO3, LT) are multifunctional crystalline materials. They exhibit quantifiable electro‐optic, acousto‐optic and non‐linear optical properties. These properties enable their use in filtering and other optical functions. Lithium niobate shows a photorefractive effect that permits its use in holographic storage. In the electrical domain, LN and LT also display piezoelectric and pyroelectric effects. They are used as substrates for piezoelectric devices and as components in pyroelectric detectors.
This article presents the two materials by detailing their crystal structures, optical properties, electrical properties, application fields and manufacturing methods. The provided data support the selection of materials for specific applications.

Crystal Structures of Lithium Niobate and Lithium Tantalate
LN belongs to the trigonal crystal system. It is classified in the 3m point group and the R3c space group with a threefold rotation axis. It is divided into near-stoichiometric lithium niobate (SLN) and congruent lithium niobate (CLN). LT also belongs to the trigonal crystal system and conforms to the ilmenite structure based on the ABO3 oxygen-octahedra framework. The crystal structure dictates the optical properties that are central to non-linear optics and electro-optical applications.
Table 1: Crystal Structure Information
|
LN |
LT |
|
|
Crystal System |
Trigonal |
Trigonal |
|
Lattice Parameters |
a = b = 5.148 Å, c = 13.863 Å |
a = 5.154 Å, c = 13.783 Å |
|
Point/Space Group |
3m point group |
C63v/R3C point group |
3 Optical Properties of Lithium Niobate and Lithium Tantalate
The crystal structures result in measurable non-linear optical coefficients. LN and LT have high quadratic non-linear coefficients that facilitate frequency doubling, mixing, summing and difference frequency generation. They possess significant electro-optic coefficients. An applied electrical field alters the refractive index. This capability makes them suitable for electro‐optic modulators and optical switches. Both materials exhibit birefringence with two distinct refractive indices. Their transparency covers wavelengths in the visible and infrared regions, which is critical for optical communication and laser applications.
Lithium niobate crystals display a photorefractive effect. Under high-intensity light, the refractive index changes unevenly. Early studies indicated that the effect reduced frequency conversion efficiency. Later, controlled irradiation or high-temperature treatment mitigated the effect. The photorefractive effect is now used in optical information processing, holographic displays, spatial modulation, optical time delay and image processing. At high light intensities, light-induced scattering may occur. A delayed response can distort information transmission.
Lithium tantalate crystals exhibit properties that are quantitatively similar to those of lithium niobate. In holographic storage research, LT is among the most tested photorefractive materials. Its photorefractive resistance is higher than that of LN by over two orders of magnitude. Both materials are employed based on the required operational parameters.
Table 2: Properties of LN and LT
|
LN |
LT |
|
|
Melting Point |
1250 °C |
1650 °C |
|
Curie Temperature |
1140 °C |
610 °C |
|
Density |
4.64 g/cm³ |
7.45 g/cm³ |
|
Mohs Hardness |
5 |
5.5–6 |
|
Spectral Range |
0.4–2.9 µm |
0.4–5.0 µm |
|
Refractive Index |
no = 2.286 |
no = 2.176 |
|
Thermal Expansion Coefficient |
a11 = 15.4×10–6/K |
αa = 1.61×10–6/K |
4 Electrical Properties of Lithium Niobate and Lithium Tantalate
Ferroelectricity and the Piezoelectric Effect
Both LN and LT fall into the category of ferroelectric crystals. They become electrically polarised when an external field is applied and retain this polarisation until a reverse field is applied. This behaviour is due to their non-centrosymmetric crystal structure. Ferroelectric crystals are used in capacitors, sensors and memory devices.
The piezoelectric effect converts external mechanical forces to electrical signals. When a force is applied, the crystal lattice distorts and generates a voltage across opposite surfaces. When the force is removed, the crystal regains its original state. This direct conversion is used in sensor applications.
LN and LT: Quantitative Piezoelectric Materials
Both lithium niobate and lithium tantalate are used in piezoelectric devices. Their electromechanical coupling coefficients exceed those of quartz. They are applied in resonators, transducers, delay lines and filters. In high-frequency communications, SAW filters based on these materials operate in fields such as mobile and satellite communications, digital signal processing, television, radar and electronic countermeasures. Their performance is confirmed by measured coupling coefficients.
A change in spontaneous polarisation with temperature constitutes the pyroelectric effect. The effect produces a voltage when the temperature rate (dT/dt) is positive or negative, depending on whether the crystal is in an open or short-circuited state.
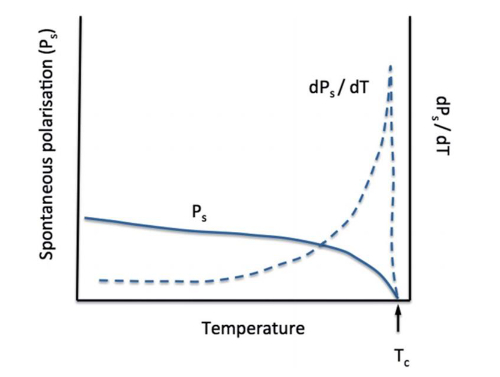
5 Applications of Lithium Niobate and Lithium Tantalate
5.1 SAW Filters
Surface acoustic wave (SAW) filters have been examined in detail. Their low insertion loss, high reliability and flexible design suit them for both analogue and digital systems. Materials for these filters require a high surface quality, measurable electromechanical coupling, low transmission loss and a small temperature coefficient. These characteristics are quantifiable during manufacture.
Resonators form the basic unit of these filters. The resonator performance directly affects overall filter performance. SAW resonators typically operate between 10 MHz and 1 GHz. They achieve insertion losses in the range of 1 to 5 dB. Lithium tantalate exhibits a higher Q-factor when used as a resonator element.
5.2 Oscillators
Oscillators convert direct current into alternating current at fixed frequencies. They exchange energy between magnetic and electric fields to sustain oscillations. Crystal oscillators employ the piezoelectric effect. Although quartz is common because of its low temperature coefficient and stability, its electromechanical coupling is limited. Research on LT wafers has led to improvements in frequency and size parameters.
5.3 Pyroelectric Detectors
Pyroelectric detectors function by dissipating heat to the environment through convection, conduction and radiation. They generate charge as electrons are adsorbed on the surface when temperature changes occur. These detectors provide high detection rates, operate at high frequencies and have fast response times. Manufacturers use single crystals of lithium niobate and lithium tantalate in these applications.

5.4 Q-Switches
Q-switch technology in lasers involves an optical switch within the cavity. This switch adjusts the Q-factor, which quantifies the quality of the optical resonator. It facilitates pulse compression and an increase in peak power. Electro-optic Q-switches, which utilise crystals such as potassium di-deuterium phosphate, lithium tantalate, lithium niobate and rubidium oxytitanate, are common in nanosecond pulsed lasers. Lithium tantalate is preferred for its consistent performance and high damage threshold.
5.5 Holographic Storage
During the 20th century, data storage devices such as magnetic tapes and optical discs faced capacity limits. Optical storage, particularly holographic grating storage, offers increased capacity. The storage capacity scales with the cube of the inverse wavelength. Holographic storage utilises the photorefractive effect in LN crystals. Practical implementation has been limited by saturation diffraction efficiency, slow response and instability. Doping LN with elements such as Fe, Mn or In can improve performance.
6 Manufacture of Lithium Niobate and Lithium Tantalate
6.1 Manufacture of Lithium Niobate
6.1.1 Production of Homogeneous Lithium Niobate
Homogeneous lithium niobate is often produced by the Czochralski pulling method. The quality of the crystals is affected by the raw material ratio, pulling speed, quality of seed crystals and crucible design. The straight pulling method employs simple equipment and allows for controlled doping.
6.1.2 Production of Near-Stoichiometric Lithium Niobate
The dual-crucible method is commonly used with a continuous feeding system. This method produces near-stoichiometric (nSLN) crystals from lithium-rich melts. However, the method is complex and the growth rate is slow. The resultant crystals have low yield and higher cost. An alternative method is lithium diffusion in CLN crystals. This diffusion occurs in a lithium-rich atmosphere controlled by temperature and time. The process yields optically homogeneous nSLN crystals if the substrate quality is high. In practice, thicker or non-z-cut substrates may develop cracks after diffusion.
6.2 Manufacture of Lithium Tantalate
6.2.1 Production of Homogeneous Lithium Tantalate
Lithium tantalate crystals of uniform composition are produced by mixing high-purity tantalum pentoxide and lithium carbonate in a molar ratio of 0.95:1. The Czochralski method is used for pulling the crystals. Quality is affected by the raw material ratio, pulling speed, seed crystal quality and crucible design. The crystals are sliced, polished and cleaned to form lithium tantalate wafers. Alternative methods such as blistering, die-guiding and temperature-gradient techniques exist but are less common because of cost and process complexity.
6.2.2 Production of Near-Stoichiometric Lithium Tantalate
Producing lithium tantalate crystals with near-stoichiometric composition is challenging. Current methods include the dual-crucible method, flux-pulling method, zone melting and gas-phase exchange equilibrium. In the dual-crucible method, additional melt is supplied continuously to maintain the composition. Although this yields homogeneous crystals, the process is complex and costly. The flux-pulling method uses a flux (commonly K₂O) to adjust the melting point. Nonetheless, the flux may incorporate into the crystal and change the melt composition during growth, making uniformity difficult. The zone melting technique uses a local heat source to create a melt zone that is moved along a seed crystal. This method conserves energy and raw material while ensuring composition uniformity, though it requires skilled operation. The gas-phase exchange equilibrium method offers control over the lithium content during growth. However, it is time-consuming and best suited for large plate samples, with difficulties in achieving large, uniform single crystals.
Table 3: Comparison of Various Methods for Producing Near-Stoichiometric Lithium Tantalate
|
Procedure |
Advantages |
Disadvantages |
|
Dual-Crucible Method |
1. Produces homogeneous, near-stoichiometric crystals. |
1. Complex process with higher cost. |
|
Flux-Pulling Method |
1. Relatively simple procedure. |
1. Flux readily incorporates into the crystal. |
|
Zone Melting Process |
1. Ensures uniform composition. |
1. Process is relatively complex. |
|
Gas-Phase Exchange Equilibrium Method |
1. Enables control of the lithium content during growth. |
1. Long processing times. |
7 Conclusion
Both lithium niobate and lithium tantalate have measurable non-linear optical and optoelectronic properties. They are utilised in filters, electro‐optic components, piezoelectric and pyroelectric devices and holographic storage. Lithium niobate is favoured for applications where higher resolution and image quality are required, whereas lithium tantalate is selected in cases where photorefractive effects must be minimised. The crystal pulling method remains the fundamental manufacturing process. Various methods for producing LT crystals offer quantifiable advantages and disadvantages. For further technical queries, please consult the experts at Stanford Advanced Materials. You may also contact them for additional details.
Product Pages:
CY0027 Lithium Tantalate Wafers (LiTaO3 Wafers)
CY0066 Lithium Niobate Wafers (LiNbO3 Wafers)
References
[1] Xiao, X., Xu, Q., Liang, S. et al. Preparation, electrical, thermal, and mechanical properties of near-stoichiometric lithium tantalate wafers. J Mater Sci: Mater Electron 33, 20668–20677 (2022). https://doi.org/10.1007/s10854-022-08878-3
[2] Kimura T, Omura M, Kishimoto Y, et al. A comparative study of acoustic wave devices utilising thin piezoelectric plates in the 3–5 GHz range. IEEE Transactions on Microwave Theory and Techniques, 2019, 67(3): 915–921.
[3] Ruby R, Gilbert S, Lee SK, et al. Novel temperature-composed, silicon SAW design for filter integration. IEEE Microwave and Wireless Components Letters, 2021, 31(6): 674–677.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators




 Chin Trento
Chin Trento



