Something You Should Know About Lithium-Ion Battery
Introduction
Lithium-ion batteries, frequently abbreviated as LIBs, are rechargeable energy storage devices that have become the standard for powering a variety of modern technologies. From smartphones and laptops to electric vehicles and renewable energy storage systems, LIBs have changed the methods by which electrical energy is utilised and stored. This article examines the role of LIBs in these sectors and provides detailed information on their operating principles, raw materials, advantages and applications.
 [1]
[1]
Figure 1. Lithium-ion battery
Operating Principles of Lithium-ion Batteries
Lithium-ion batteries operate on an electrochemical process. They function by moving lithium ions (Li+) between two primary components: the anode and the cathode. During the charging phase, lithium ions are removed from the cathode and stored in the anode, thereby creating a potential difference. During the discharging phase, as the battery supplies power, these ions pass through an electrolyte solution back to the cathode.
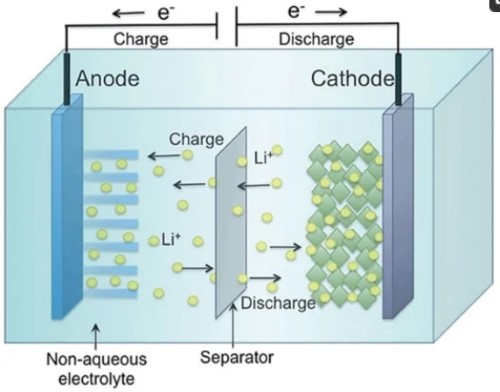 [2]
[2]
Figure 2. Structure of a lithium-ion battery
Materials in Lithium-ion Batteries
In the electrochemical process, different LIB materials are selected and developed to maximise battery performance, energy density and overall efficiency. The following are the main components of lithium-ion battery materials:
-Anode Materials
Anodes in LIBs typically consist of materials that can absorb and release lithium ions during charging and discharging cycles. Common anode materials include graphite and silicon.
lGraphite is the most common anode material in commercial lithium-ion batteries due to its stability and electrochemical performance.
lSilicon offers a higher theoretical capacity for storing lithium compared to graphite. The expansion and contraction of silicon during lithiation and delithiation cycles poses a technical challenge.
-Cathode Materials:
Cathodes determine battery voltage and capacity. Different cathode materials present varying voltage plateaus and energy densities.
lLithium cobalt oxide (LiCoO2) was frequently used in early LIBs, particularly in consumer electronics. It provides an acceptable energy density, although its use in high-capacity and high-power applications is limited owing to safety concerns and cost.
lLithium iron phosphate (LiFePO4) is known for its safety and long cycle life. It is used in electric vehicles and renewable energy storage systems where safety and durability are necessary.
lLithium nickel cobalt manganese oxide (NCM) and lithium nickel cobalt aluminium oxide (NCA) cathodes are frequently employed in batteries for electric vehicles. They balance energy density with power density.
lLithium manganese oxide (LMO) is valued for its thermal stability and safety, and is suited for applications that require effective temperature control.
-Electrolyte:
The electrolyte is the conducting medium that enables the movement of lithium ions between the anode and cathode during charging and discharging. Common electrolytes include lithium salts dissolved in organic solvents, although solid-state electrolytes are also under development to improve safety and energy density.
-Separators:
Separators are porous membranes that physically separate the anode and cathode while allowing the transfer of lithium ions. They are usually composed of polyethylene (PE) or polypropylene (PP) and help to prevent short circuits and enhance safety.
Advantages of Lithium-ion Batteries
Lithium-ion batteries are used in various applications because of the following features:
lEfficiency: LIBs offer a high energy density, are rechargeable and exhibit a low self-discharge rate.
lDurability: LIBs operate across a wide temperature range, from sub-zero conditions to high temperatures. They undergo many charge and discharge cycles before a significant reduction in capacity is observed.
lSustainability: LIBs produce fewer emissions and possess a lower environmental impact compared to some alternative energy storage solutions.
lSafety: Modern LIBs incorporate safety features such as thermal protection, overcharge protection and integrated battery management systems (BMS) to monitor and control battery performance and safety.
Applications of Lithium-ion Batteries
LIBs have been implemented in various sectors as a result of their characteristics:
lConsumer Electronics: LIBs supply smartphones, tablets, laptops, cameras and other portable devices with the power required for modern connectivity.
lElectric Vehicles (EVs): LIBs serve as the primary energy storage system in electric vehicles. They store large quantities of energy and deliver it efficiently, thereby supporting the growth of the electric vehicle market.
lRenewable Energy Storage: LIBs are essential for storing electricity generated from renewable sources such as solar panels and wind turbines. The stored energy can be deployed during periods of low generation or at peak demand.
lAerospace: LIBs are used in aerospace applications, for instance in satellites, spacecraft and unmanned aerial vehicles (UAVs), where high energy density and reliable performance are required.
lMedical Devices: LIBs are employed in medical devices to ensure that critical equipment, such as implantable cardioverter-defibrillators (ICDs) and portable medical monitors, remain operational when required.
Conclusion
In summary, lithium-ion batteries provide a viable solution for clean, efficient and sustainable energy storage. They change the way in which devices, vehicles and renewable energy systems receive power, supported by their high energy density, extended service life and versatility.
Stanford Advanced Materials (SAM) supplies a range of lithium-ion battery materials, including lithium nickel cobalt manganese oxide (NCM), lithium nickel cobalt aluminium oxide (NCA), lithium cobalt oxide (LCO) and lithium iron phosphate (LFP). Please contact us if interested.
Reference:
[1] Chandler, D. L. (23/03/2023). Study indicates cost reduction in lithium-ion batteries. MIT News. Retrieved on 12/09/2023, from https://news.mit.edu/2021/lithium-ion-battery-costs-0323
[2] Ghiji, M.; Novozhilov, V.; Moinuddin, K.; Joseph, P.; Burch, I.; Suendermann, B.; Gamble, G. A Review of Lithium-Ion Battery Fire Suppression. Energies 2020, 13, 5 117. https://doi.org/10.3390/en13195117

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Chin Trento
Chin Trento



