An Overview Of Calcium Carbonate Crystal Substrates
Introduction
Calcium Carbonate Crystal Substrates represent a new field in materials science. These substrates are receiving attention owing to their distinct properties and potential applications in various technological and environmental fields.

This article discusses the features, synthesis, applications and future prospects of calcium carbonate crystal substrates. It examines their role in materials science.
Characteristics of Calcium Carbonate Crystal Substrates
Calcium Carbonate (CaCO₃) is a commonly occurring substance in rocks, eggshells, pearls and marine organisms such as corals. In its pure crystal substrate form it shows high biocompatibility, low thermal conductivity and environmental stability. These properties support its use in scientific and industrial applications.
 [1]
[1]
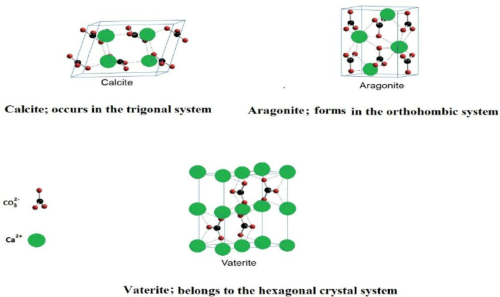
Calcium carbonate crystallises in three polymorphic forms: Calcite, Aragonite and Vaterite. Each form has a specific crystal structure and quantifiable properties.
- Calcite is the most stable form. It exhibits marked birefringence and transmits visible light. These traits are useful in optics.
- Aragonite shows high pressure and temperature stability. It is chosen for applications that require strength.
- Vaterite is less stable and is highly reactive. It is useful in rapid biomineralisation processes.
Synthesis of Calcium Carbonate Crystal Substrates
The synthesis of calcium carbonate crystal substrates can be achieved by several methods, including chemical vapour deposition, hydrothermal synthesis and biomimetic strategies.
- Chemical Vapour Deposition: This method produces high-purity calcium carbonate by depositing evaporated compounds onto a substrate. Thin layers form uniformly and can be controlled.
- Hydrothermal Synthesis: Under high pressure and temperature, crystals are synthesised that are purer and have a defined crystalline structure. This process is used for applications that require precision.
 [2]
[2]
- Biomimetic Synthesis: This approach replicates biological processes to form crystals at ambient temperature and pressure. Organic molecules are used as templates to control crystal growth. This method results in controlled morphology and size for specific applications.
Environmental factors such as temperature, pH and the presence of impurities or additives play a crucial role in determining the crystal form and quality. For example, adding magnesium ions may inhibit calcite formation and promote aragonite. Understanding these factors is necessary to design a synthesis process that yields crystals with specified properties.
Further reading: Was ist chemische Gasphasenabscheidung (CVD)?
Applications of Calcium Carbonate Crystal Substrates
Calcium carbonate crystal substrates have a broad range of applications owing to their measured properties.
- In Optics: The birefringence of calcite is used in the manufacture of polarisers and waveplates. These components are incorporated in various optical instruments and devices.
- In Biomedicine: Owing to its biocompatibility and solubility, calcium carbonate is applied in drug delivery systems and as scaffolds for bone regeneration. It is used in formulations that allow controlled release of therapeutics.
- In Environmental Sciences: The ability of calcium carbonate to absorb and mineralise CO2 positions it as a medium for carbon capture and storage (CCS) technologies. These processes aim to reduce greenhouse gas emissions.
- Additionally, in the field of catalysis, calcium carbonate substrates are employed to develop new catalytic processes that are more efficient and environmentally friendly.
Challenges and Future Prospects
Despite their potential, the use of calcium carbonate crystal substrates faces several challenges. The main issue is the control of crystal purity and structure during synthesis. This control is critical for applications that require precision, for example in optics and electronics. Furthermore, the scalability of synthesis methods must be addressed to meet industrial demands.
Research aims to improve the quality and control of synthetic calcium carbonate crystals and to broaden their range of applications.
Advances in nanotechnology may lead to the development of nano-structured calcium carbonate substrates with increased surface area and reactivity. This may open new possibilities in catalysis and environmental applications.
Another area for study is the integration of calcium carbonate substrates with other materials to form composite materials. Such composites may combine the measured properties of calcium carbonate with those of other materials such as polymers or metals. This integration is pursued to create materials for a wide spectrum of applications.
Conclusion
Calcium carbonate crystal substrates represent an emerging field in materials science. Owing to their distinct properties and wide-ranging applicability, they present quantifiable opportunities. As research progresses to unlock their full potential, their impact on technology and environmental management is expected to increase.
Table 1. Overview of Calcium Carbonate Crystal Substrates
|
Category |
Details |
|
Properties |
- High biocompatibility - Low thermal conductivity - Environmental stability |
|
Polymorphic Forms |
- Calcite: Most stable; exhibits marked birefringence; transparent to visible light - Aragonite: Exhibits high pressure and temperature stability; chosen for high strength applications - Vaterite: Less stable; highly reactive; useful for rapid biomineralisation |
|
Synthesis Methods |
- Chemical Vapour Deposition: Produces high-purity, controllable thin layers - Hydrothermal Synthesis: Utilises high pressure and temperature to achieve a defined structure - Biomimetic Synthesis: Replicates biological processes and incorporates organic components |
|
Applications |
- Optics: Manufacture of polarisers and waveplates - Biomedicine: Drug delivery systems and scaffolds for bone regeneration - Environmental Sciences: Carbon capture and storage (CCS), catalysis |
|
Challenges |
- Control of crystal purity and structure - Scalability of synthesis methods |
|
Future Prospects |
- Advances in nanotechnology to enhance surface area and reactivity - Development of composites that combine calcium carbonate with polymers or metals |
Stanford Advanced Materials (SAM) offers high-quality calcium carbonate crystals at competitive prices. As a leading supplier, SAM delivers over 3 000 advanced materials to key sectors such as aerospace, engineering, medicine and energy. Customised modifications are available. For further information, please visit our homepage.
Reference:
[1] Maleki Dizaj, Solmaz & Barzegar-Jalali, M. & Zarrintan, M. & Adibkia, Khosro & Lotfipour, Farzaneh. (2015). Calciumcarbonat-Nanopartikel; Potential in Bone and Dental Disorders. Pharmazeutische Wissenschaften. 20. 175-182. 10.5681/PS.2015.008.
[2] Tatarchuk, Tetiana & Peter, Amalthi & Al-Najar, Basma & Vijaya, Judith & Bououdina, Mohamed. (2018). Photocatalysis: Activity of Nanomaterials. 10.1002/9783527808854.ch8.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators




 Chin Trento
Chin Trento


