Inorganic Aerogels: From Nanoporous Materials to High-Efficiency Thermal Insulation Solutions
1 Fundamental Concepts and Remarkable Properties of Aerogels
Aerogel is a three-dimensional nanoporous solid material. It is prepared through sol-gel synthesis combined with specialised drying techniques, such as supercritical drying or atmospheric drying. Often referred to as “solidified smoke,” its uniqueness lies in its internal porosity, which exceeds 90%. This means air occupies the vast majority of the material's space. The structure forms a nano-scale pore system, supported by a solid framework and filled with gas. This unique architecture gives aerogels several exceptional properties. These include ultra-low thermal conductivity (0.012–0.024 W/(m·K)), ultra-high specific surface area, low density, and outstanding functional design flexibility.
Chemically, aerogels are primarily categorised into three types. These include inorganic aerogels (such as silica aerogel and alumina aerogel), organic aerogels (such as polyimide aerogel and cellulose aerogel), and carbon-based aerogels (such as graphene aerogel and carbon nanotube aerogel). This diversity enables aerogels to adapt to diverse application demands. Uses range from thermal protection in extreme environments to energy-saving insulation in daily life. This demonstrates their immense potential across numerous fields.
This article focuses on inorganic aerogels, primarily silica and alumina-based variants, which have proven themselves in thermal protection, energy-saving insulation, and other high-performance applications.

Fig. 1 Aerogel
2 Properties of Aerogels Made from Different Inorganic Materials
2.1 Silica Aerogel: A Multifunctional Material with Ultralow Thermal Conductivity
Silica aerogel is a lightweight, porous, amorphous material with outstanding exothermic thermal insulation properties. Its porosity can reach 80–99.8%, with pore sizes typically distributed between 1 and 100 nm. It exhibits a specific surface area of 200–1000 m2/g and a density as low as 3 kg/m3. At room temperature, its thermal conductivity is as low as 0.012 W/(m·K)—two to three orders of magnitude lower than conventional inorganic insulating materials and even below that of static air. Even at 800°C, its thermal conductivity remains only 0.043 W/(m·K). It exhibits stable properties at high temperatures without decomposition, making it an environmentally friendly material.
Due to the significantly reduced sound velocity within silica aerogel, it serves as an acoustic delay or high-temperature sound insulation material. Its wide range of acoustic impedance (10^3–10^7 kg/(m2·s)) enables its use as an acoustic impedance coupling material to amplify sound intensity. Furthermore, due to the nano-network structure of silica aerogel, dopants exist as nanocrystals within it, exhibiting strong visible light emission. This provides compelling evidence for quantum confinement luminescence in porous silicon. Leveraging the silica aerogel structure and the nonlinear optical effects of C60, novel laser protective lenses can be further developed.
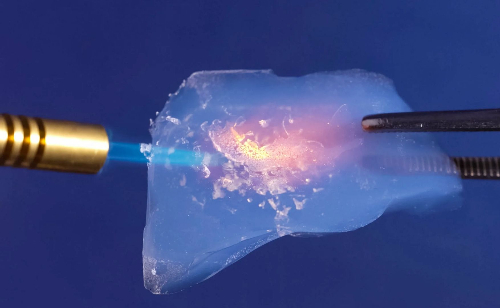
Fig. 2 Silica Aerogel Heat Resistance Test
2.2 Alumina Aerogel: A High-Temperature Stable Insulator
Alumina aerogel materials are novel inorganic non-metallic materials primarily composed of alumina, featuring a core structure of a nanoporous network. Characterised by high specific surface area, high porosity, and low density, it is an exceptional porous material with outstanding thermal insulation properties. Its thermal conductivity is significantly lower than that of traditional insulation materials, effectively blocking heat transfer.
The most prominent features of alumina aerogel are its extremely high specific surface area and low density. Research indicates that through optimised preparation techniques, its specific surface area can reach up to 744.5 m2/g, while its density can drop as low as 0.063 g/cm3. This material forms a three-dimensional network structure composed of nanoparticles, internally filled with nanoscale pores. This confers high porosity, with pore diameters typically ranging from 10 to 100 nanometres and pore volumes reaching 0.4–0.9 cm3/g. These structural features collectively confer exceptional thermal insulation properties to alumina aerogel. At ambient temperature (30°C), its thermal conductivity can be as low as 0.029 W/(m·K). Even under high-temperature conditions (e.g., 1000°C), the thermal conductivity remains only 0.0685 W/(m·K).
Alumina aerogel also demonstrates outstanding chemical and thermal stability. Compared to silica aerogel, it exhibits superior high-temperature resistance, maintaining its nanoporous structure well even at 1000°C. Studies also reveal that after 2 hours of heat treatment at 1200°C, its specific surface area remains at 153.45 m2/g, with no significant changes to its leaf-like porous structure, demonstrating outstanding high-temperature stability. Doping with heteroatoms such as strontium, lanthanum, and silicon can further suppress phase transitions and grain sintering at elevated temperatures. For instance, silicon-doped samples exhibit a specific surface area of 146 m2/g after 1200°C heat treatment, thereby extending the upper operating temperature limit to 1600°C.
2.3 Aluminosilicate Composite Aerogel: Enhanced Toughness and Ultra-High Temperature Resistance
Aluminium silicate aerogels have garnered significant attention due to their exceptional high-temperature resistance and mechanical strength. While traditional silica aerogels exhibit extremely low thermal conductivity, they suffer from structural collapse and performance degradation at elevated temperatures (typically exceeding 800°C). Conversely, pure alumina aerogels, though capable of withstanding higher temperatures, often face stability challenges stemming from phase transitions.
By incorporating an alumina phase into silica aerogel, aluminosilicate-based aerogels successfully extend the material's temperature tolerance range to 1200–1400°C while maintaining a low thermal conductivity at elevated temperatures. This composite material combines the nanoporous structure of silica with the high-temperature stability of alumina. The incorporation of aluminosilicate fibres as reinforcing phases effectively addresses the inherent brittleness and poor mechanical properties of traditional aerogels.

Fig. 3 Aluminium Silicate Aerogel Composite Board Insulation Material
3 Preparation Techniques and Challenges for Aerogels
3.1 Silica Aerogel: Precursor Routes and Industrial Scalability
Trimethyl orthosilicate (TMOS) and tetraethyl orthosilicate (TEOS) are the most classic silicon sources for preparing high-purity, high-performance silica aerogels. Their synthesis primarily involves two key reactions: hydrolysis and condensation. Hydrolysis generates active silanol groups, which are then condensed to form a stable three-dimensional Si-O-Si network framework. This approach offers advantages of high product purity and structural tunability; however, its drawbacks include the inherent toxicity of the precursors and relatively high raw material costs. Starting from these precursors, a series of refined processes—including gelation, ageing, solvent exchange, and supercritical drying—ultimately yield structurally complete, pure silica aerogels.
Silica sol sources, stable colloids formed by dispersing nanoscale silica particles in water or solvents, represent another practical silicon source for silica aerogel synthesis. This process bypasses some hydrolysis steps, directly utilising preformed nanoparticles in the sol as fundamental structural units to construct a three-dimensional network through concentration and polycondensation. This method offers relatively simplified processing and lower raw material toxicity. Aerogels prepared via this route also require subsequent treatments such as gelation, ageing, solvent exchange, and supercritical drying. The resulting aerogel materials can achieve excellent levels of purity and performance.
Water glass (sodium silicate solution) stands out as an ideal choice for large-scale industrial production of silica aerogels due to its significant advantages of low cost and readily available raw materials. However, a core challenge in its preparation process lies in the introduction of impurities such as sodium ions (Na+) into the gel network. These typically require rigorous ion exchange and extensive solvent washing and replacement steps for removal, making the process relatively cumbersome. Despite these purification challenges, optimised subsequent treatments can still effectively enhance the purity and overall performance of the final aerogel product, enabling it to demonstrate strong competitiveness in cost-sensitive application areas.
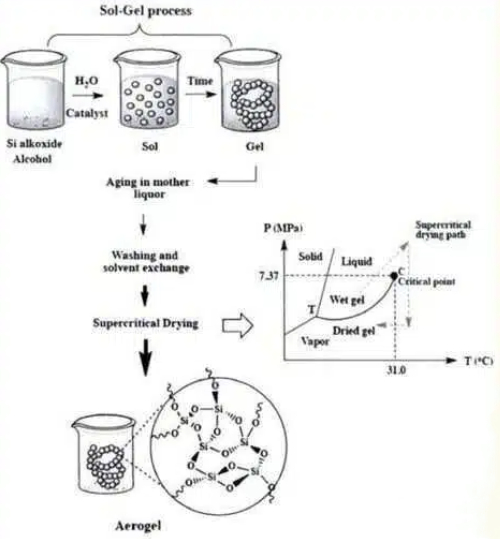
Fig. 4 Preparation of Silica Aerogel
3.2 Alumina Aerogel: Precursor Selection and Stability Challenges
The preparation of high-performance alumina aerogels mainly uses the alcoholate hydrolysis route, which is the most established method for producing high-purity products. This process utilises precursors such as aluminium sec-butoxide or aluminium isopropoxide. It involves tightly controlled hydrolysis and condensation reactions that create an interconnected Al-O-Al network. Afterward, supercritical drying produces aerogels with well-defined nanostructures and high specific surface areas. While this approach ensures a superior pore structure and purity, its practical use is limited by the high cost and notable moisture sensitivity of the precursors.
To overcome economic challenges, the inorganic aluminium salt method offers a practical alternative. This method uses cost-effective precursors such as aluminium chloride or nitrate and employs gelation promoters like propylene oxide to influence reaction speed. Although it is simpler to operate and has low raw material costs, this approach introduces anionic impurities, requiring extensive purification through repeated washing. If these residues are not adequately removed, they can significantly weaken the thermal stability of the resulting aerogel.
Improving high-temperature performance is a major area of research, with elemental doping becoming an essential strategy. Adding stabilisers like lanthanum, silicon, or strontium can effectively reduce harmful phase changes, especially the γ→α transition, and prevent grain coarsening at higher temperatures. Optimised doping mixtures allow for the retention of specific surface areas greater than 150 m2/g after exposure to 1200°C, thus raising the maximum service temperature to around 1600°C.
The drying method is crucial for maintaining the structure of the final product. Supercritical drying is the standard technique, as it nearly eliminates capillary stresses while removing the solvent, preserving nanoscale architecture. Alternatively, atmospheric pressure drying techniques have emerged that utilise surface functionalisation methods, such as silanisation treatments. These treatments give the gel network hydrophobic properties. This improvement allows for successful drying under normal conditions while keeping the structural integrity intact, providing a promising option for large-scale manufacturing.
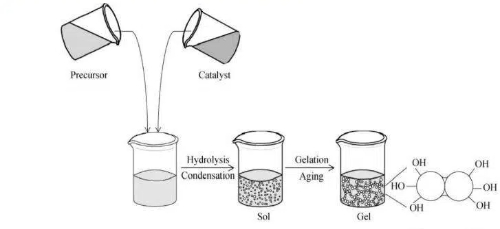
Fig. 5 Sol-Gel Process
3.3 Aluminosilicate Aerogel: A Composite Design Strategy
Fibre-reinforced framework technology serves as the core solution for enhancing the mechanical and thermal properties of aerogels. This method utilises pre-fabricated aluminosilicate or mullite fibres as a three-dimensional framework, employing a sol-gel process to in situ construct a nanoporous aerogel matrix within the fibre network. This composite configuration—a “fibre load-bearing skeleton + aerogel thermal insulation filler”—combines the toughness and strength of fibres with the thermal insulation properties of aerogel, successfully overcoming the brittleness of traditional silica aerogels.
Interfacial control is pivotal in determining composite performance. Research confirms that precisely regulating the sol-gel process pH environment—such as maintaining weakly alkaline conditions around pH=8—is critical. Under these optimised conditions, the aerogel precursor deposits more uniformly and adheres firmly to the fibre surface, significantly enhancing interfacial bonding strength. This manifests macroscopically as a marked improvement in the material's overall mechanical strength.
Mullite phase reinforcement represents a strategy for optimising high-temperature performance. Compared to conventional aluminosilicate fibres, mullite fibres inherently exhibit better thermal stability and reduced high-temperature creep. Utilising mullite as the reinforcing phase suppresses shrinkage and sintering phenomena in composites exposed to extreme environments exceeding 1000°C. This enables the material to maintain structural integrity and thermal insulation properties during prolonged high-temperature service.
4 Application Fields of Aerogels Made from Different Materials
Silica aerogel, as the most representative nanoporous material, demonstrates performance advantages in the medium-to-low temperature range below 800°C. Its thermal conductivity at room temperature can be as low as 0.018–0.023 W/(m·K). Combined with mature preparation techniques, it finds extensive applications in building energy efficiency and industrial pipeline insulation. Particularly in weight- and space-sensitive applications like thermal insulation for new energy vehicle battery packs and filling materials for outdoor gear, its lightweight nature complements its ultra-low thermal conductivity. Additionally, its Class A non-combustibility and up to 99% water repellency make it highly effective in building envelopes demanding strict fire safety and moisture resistance.
Alumina aerogel demonstrates unique value across broader temperature ranges, operating effectively at 1000-1300°C. This bridges the performance gap between silica aerogel and traditional refractory materials. By doping stabilising elements such as lanthanum and silicon, phase transitions and grain growth at high temperatures can be significantly suppressed. This allows the material to maintain a specific surface area exceeding 150 m²/g even after heat treatment at 1200°C. This characteristic makes it an ideal choice for thermal insulation in high-temperature industrial furnace linings and auxiliary insulation layers in aerospace thermal protection systems, playing a vital role in energy-saving upgrades across industries such as steel, cement, and ceramics.
Through a “fibre skeleton-aerogel matrix” composite structure design, aluminium silicate composite aerogel successfully overcomes the brittleness limitations of traditional aerogels while extending its temperature tolerance to 1200-1400°C. This unique structure maintains excellent thermal insulation while significantly improving mechanical properties, achieving compressive strength exceeding 0.46 MPa and linear shrinkage below 8% at 1200°C. These characteristics make it a critical material for extreme environments, such as missile servo compartment heat shields, aviation engine compartment insulation, and high-temperature industrial valve gaskets. It holds an irreplaceable position in aerospace, military equipment, and other fields.
Table 1 Major Aerogel Types and Their Comparative Characteristics
|
Aerogel Type |
Key Characteristics |
Temperature Limit |
Representative Applications |
|
Silica Aerogel |
Ultra-low thermal conductivity, high specific surface area |
~800℃ |
Building insulation, industrial piping |
|
Alumina Aerogel |
Stable at medium to high temperatures |
~1000℃ |
High-temperature furnace insulation |
|
Aluminosilicate Composite Aerogel |
High-temperature stability with excellent mechanical properties |
1100-1400℃ |
Aerospace and military equipment |
|
Carbon-Based Aerogel |
Electrically conductive with a high specific surface area |
~600℃ (Inert atmosphere) |
Battery electrodes and adsorbent materials |
5 Conclusion
Inorganic aerogels, as a class of advanced materials featuring three-dimensional nanoporous structures, demonstrate immense application potential across multiple fields due to their unique properties. This paper systematically analyses the material characteristics, preparation processes, and application prospects of three primary inorganic aerogels.
From a material properties perspective, silica, alumina, and aluminosilicate-based aerogels form a complete performance spectrum: Silica aerogels exhibit outstanding thermal insulation properties below 800°C, with a thermal conductivity as low as 0.012 W/(m·K) at room temperature, while also demonstrating special value in acoustics and nonlinear optics; Alumina aerogels, through optimised preparation techniques, achieve specific surface areas up to 744.5 m²/g and maintain structural stability at 1000–1300°C, filling a technical gap in medium-to-high-temperature insulation materials. Aluminosilicate composite aerogels, through a "fibre-reinforced aerogel matrix" composite design, elevate their temperature tolerance to 1400°C while significantly enhancing mechanical properties, thereby resolving the brittleness inherent in conventional aerogels. In terms of preparation techniques, each aerogel exhibits distinct characteristics: silica aerogel employs three process routes—orthosilicate, sol-gel, and water glass—balancing purity, cost, and scalability; alumina aerogel employs the alcoholate method and inorganic alumina salt method to meet high-purity and low-cost requirements respectively; while aluminosilicate composite aerogel achieves performance enhancement through fibre reinforcement, interfacial regulation, and the introduction of the mullite phase. In application domains, these materials exhibit distinct specialisations: silica aerogel dominates medium-to-low temperature sectors like building energy efficiency, industrial piping, and new energy vehicles; alumina aerogel plays a critical role in high-temperature industrial furnaces and aerospace thermal protection systems; while aluminosilicate composite aerogel holds value in extreme environments such as aerospace and military equipment.
Looking forward, inorganic aerogel development will advance toward multifunctionality, intelligence, and sustainability. At Stanford Advanced Materials (SAM), we supply high-quality aerogels, including silica, alumina, and aluminosilicate-based variants, to support industries from energy efficiency to aerospace, driving innovation and contributing to a greener, low-carbon future.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us
 Dr. Samuel R. Matthews
Dr. Samuel R. Matthews


