Recreating Liquid Metal From Terminator With Galium
In the field of visual effects, the transformations of liquid metal have made a lasting impact due to their distinct visual presentation. One well‐documented example is the character T1000 in the film Terminator 2 (1991), in which advanced CGI was used to create the metallic form of the character. For many years, the liquid metal effect of the T1000 was regarded as an exceptional achievement in visual effects. Recently, however, an experiment was conducted to reproduce this effect using actual metal – specifically, the metal Gallium.
In this experiment, the creative team, Corridor Crew, utilised the inherent properties of Gallium to reproduce the liquid metal effect seen in the T1000 sequence. Gallium was chosen because it has a low melting point and is non‐toxic, thus permitting controlled melting and reshaping in a safe environment. By combining 3D printing, silicone moulds and precise heating techniques, the team achieved the liquid metal effect without the use of CGI. This project was executed with the support of Stanford Advanced Materials (SAM), which provided the Gallium necessary for the experimental work.
Unique Properties of Gallium
The successful use of Gallium in this experiment is based on several physical and chemical properties that distinguish it from other metals:
- Low melting point: Gallium melts at 29.8 °C (85.6 °F), a temperature slightly above typical room temperature. Consequently, Gallium melts with minimal heat input, even when held. This low melting point enables experiments that require strict temperature control. In contrast to metals that need high temperatures, Gallium permits repeated melting and solidification, as the Corridor Crew experiment demonstrates.
- Non‐toxic: Unlike mercury, which is toxic, Gallium can be handled safely in small quantities. The non‐toxic nature of Gallium was essential to the experiment, allowing the team to focus on generating the required effect without posing a health risk. For further details, please refer to The Safety of Gallium.
- Industrial versatility: Gallium is used in a number of industries beyond visual effects. It plays a role in electronics and technology, particularly in semiconductors, LEDs and high-performance devices. Compounds such as Galliumnitride (GaN) are employed in high-speed transistors, power devices and 5G telecommunications equipment. Accordingly, Gallium-based materials are increasingly used in advanced technology applications instead of conventional silicon-based materials.
- Reactivity and stability: In its solid state, Gallium is stable and resistant to oxidation, which facilitates handling and storage. However, it reacts in a distinct manner with certain metals, especially aluminium, thereby creating potential for specialised applications and further study.
Gallium in the Special Effects Experiment
In their experiment, the Corridor Crew team utilised the properties of Gallium to replicate the liquid metal effect observed in Terminator 2. In order to achieve this effect, the team followed several steps: 3D printing, mould preparation and careful heating to melt and reform the metal.
Creating the mould: Initially, a 3D scan of a head was produced. This scan was printed and served as the basis for a silicone mould. In the mould, intricate details were recorded so that the final Gallium casting accurately reflected the intended design.

Casting with Gallium: Once the mould was prepared, Gallium was heated to a liquid state and poured into the mould to form a metal replica. The low melting point meant that minimal heating was required to liquefy the Gallium and fill the mould; this ensured safe and manageable handling. The solidified casting retained the detailed structure of the mould, producing a metal form with high fidelity.

Melting and filming: Following the casting process, the team commenced the visual effects procedure by applying heat guns to the casting. As the Gallium absorbed heat, melting began at the outer edges. The melting process was recorded carefully in order to document the deconstruction effect, whereby the metal shifts from a solid state to a liquid state before reshaping.

Reversal of the filmed material: During post-production, the recorded video was reversed, thereby creating the impression that the liquid metal solidifies into a fixed object. This reversal was necessary to replicate the T1000 effect, in which the Gallium exhibited a controlled fluid behaviour that is not easily achieved through CGI alone.
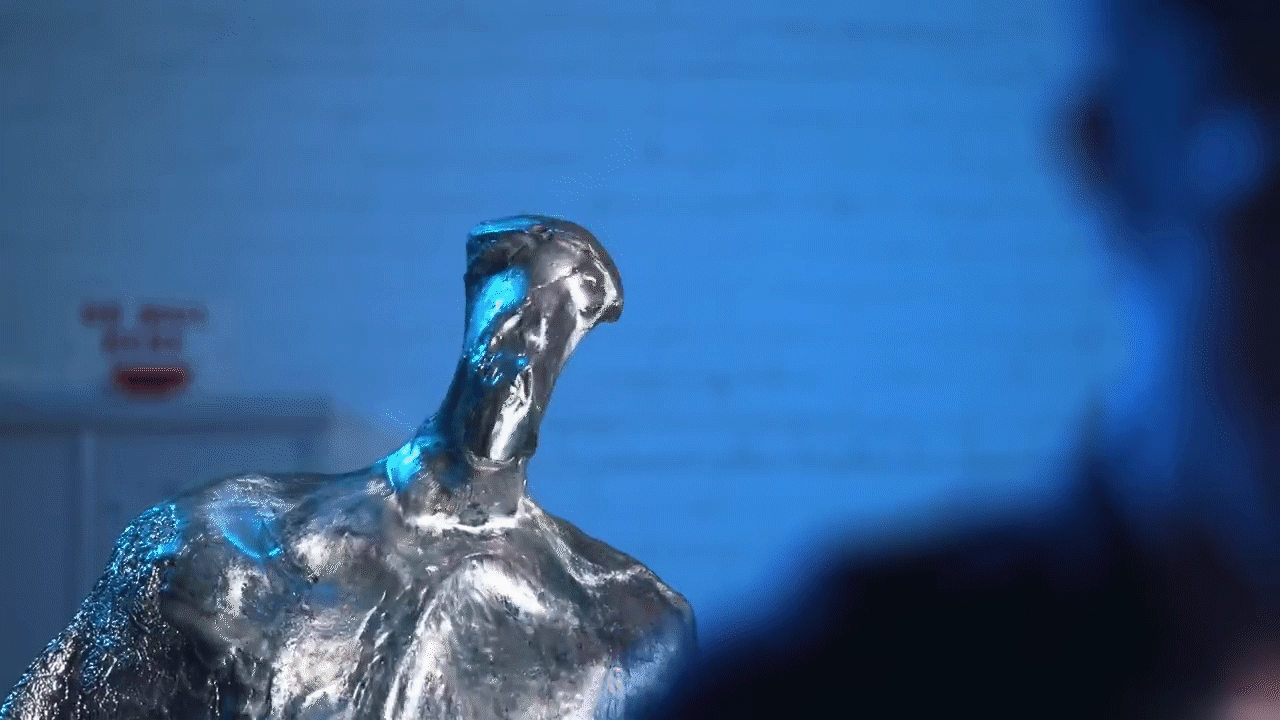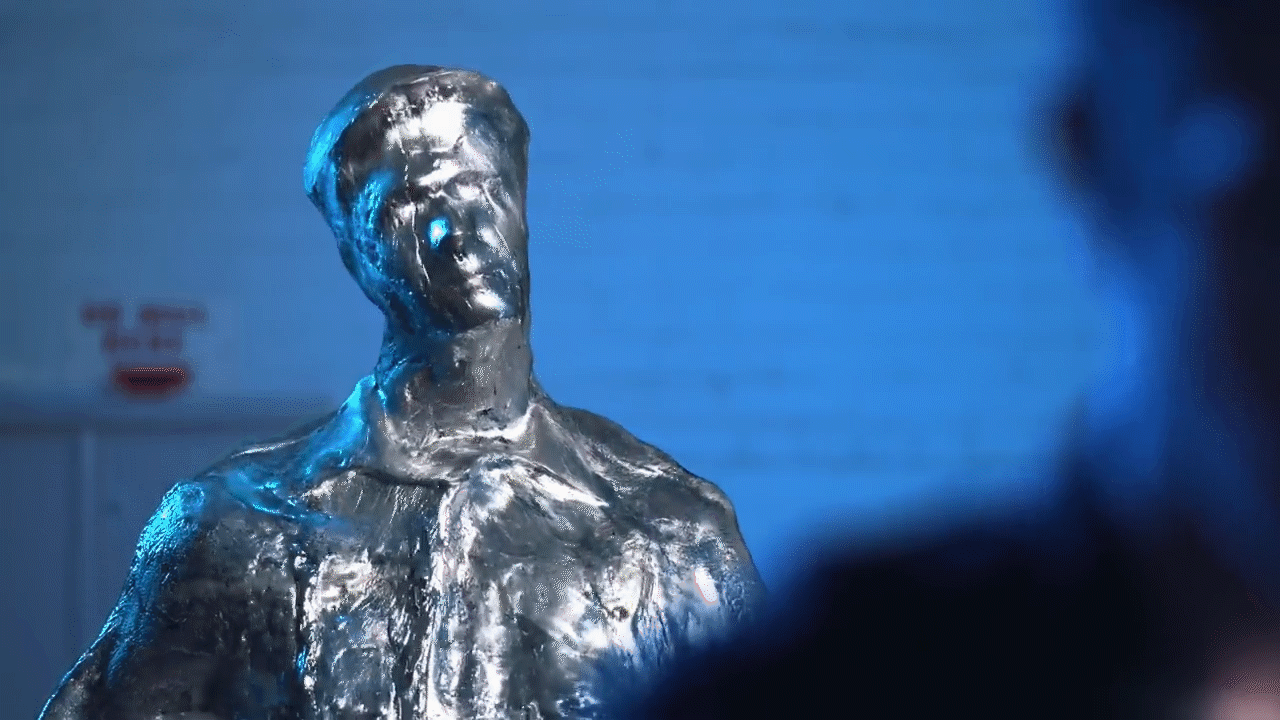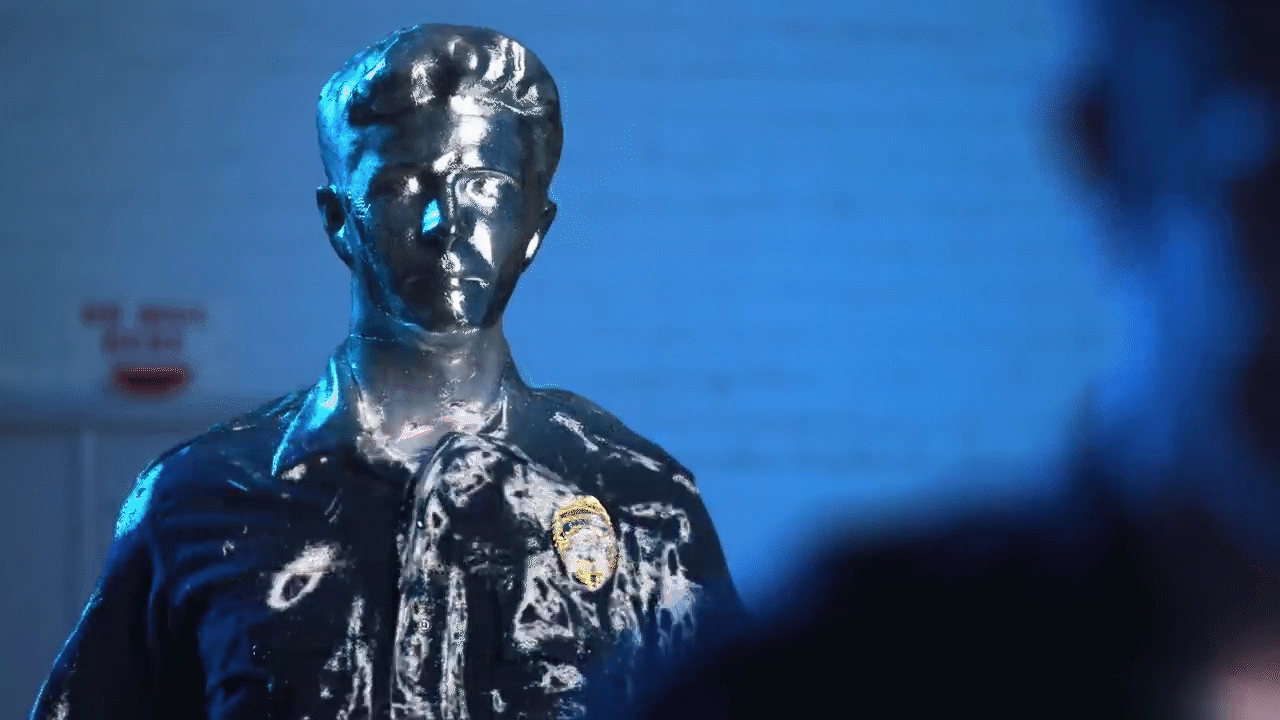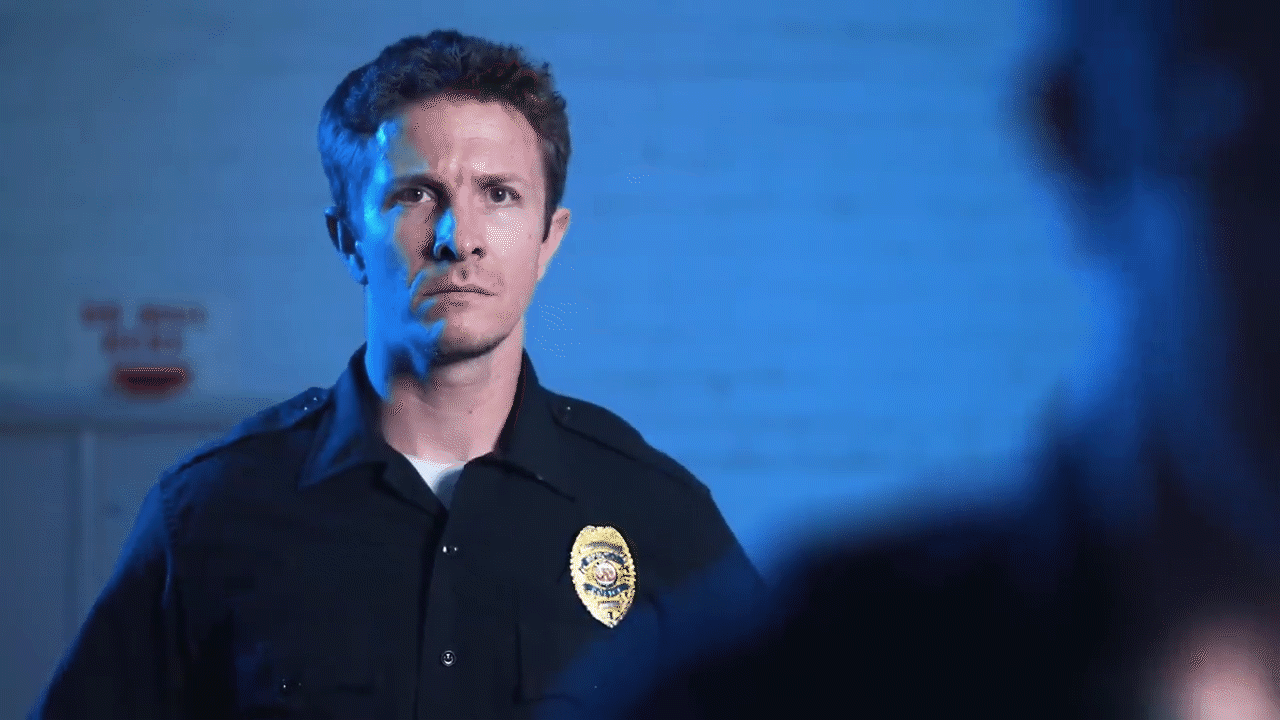
Challenges and Solutions
During the experiment, the Corridor Crew encountered issues specific to working with actual metal. Controlling the melting rate of Gallium required careful monitoring to ensure even heat distribution and to prevent the metal from collapsing too quickly or unevenly. Given that Gallium exhibits stability under controlled conditions, the desired outcome was achieved. The team also had to stabilise the casting and prevent sudden movements to maintain continuity; this approach was critical in documenting the effect accurately.
In conclusion, the inherent properties of Gallium provided an effective alternative to CGI, achieving a liquid metal effect through real material behaviour.
To view the complete process, please watch the full video here: https://youtu.be/40kkKLQfeMA
Future Applications of Gallium
The properties of Gallium made it a suitable candidate for the liquid metal experiment conducted by the Corridor Crew. However, this adaptable metal offers potential applications beyond visual effects. Its attributes – such as a low melting point, non‐toxicity and the ability to form compounds – make Gallium a valuable substance across several scientific and industrial domains.
Electronics and Telecommunications: Gallium compounds, particularly Gallium Nitride (GaN), play a significant part in the development of advanced electronics. GaN transistors are efficient and are used in high-speed circuitry and applications that demand higher power densities compared with conventional silicon transistors. This characteristic is important for power devices and high-frequency applications, including 5G telecommunications, where speed and precision are essential. Consequently, GaN-based technology has enabled the production of smaller and more efficient electronic components.
Renewable Energy: Gallium arsenide (GaAs) is acknowledged for its ability to convert sunlight into electricity efficiently. GaAs-based solar cells are employed in aerospace and other demanding environments where both durability and energy conversion efficiency are required. In this way, Gallium contributes to the advancement of solar energy technology and the creation of longer-lasting energy solutions.
Medical and Chemical Research: Owing to its biocompatibility and distinct reactivity, Gallium is being investigated for potential medical uses, including targeted cancer treatments and specific imaging techniques. In addition, the catalytic properties of Gallium may assist in developing new environmentally friendly industrial processes.
Educational and Demonstrative Applications: Because Gallium is non‐toxic and has a low melting point, it is well suited for educational demonstrations and practical projects. It provides a safe and interactive method for studying the properties of metals, making it appropriate for classroom instruction, museum displays and media production. Its ability to exist in both liquid and solid states offers observers a factual insight into materials science.
Conclusion
The combination of properties exhibited by Gallium – namely, its low melting point, non‐toxicity, stability and capacity to form compounds – demonstrates its versatility and potential in various applications. The experiment by Corridor Crew has confirmed that Gallium can reproduce a liquid metal effect without relying on CGI, thereby providing an alternative approach based on actual material behaviour.
The contribution of Stanford Advanced Materials to this project illustrates how scientific inquiry and practical application may be integrated to explore new possibilities in material usage. The role of Gallium extends into electronics, renewable energy, medical research and education, indicating that this metal has significant potential for future developments. In both applied and experimental contexts, Gallium offers scientists, engineers and creative practitioners new opportunities to advance the study of materials.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us

 Chin Trento
Chin Trento


