How To Analyze The HA Content In The Fermentation Broth?
Introduction
Hyaluronic acid (HA) is a naturally occurring polysaccharide that finds frequent application in the pharmaceutical, cosmetic and biomedical industries. Its production generally involves the fermentation of bacterial strains. Analysis of HA content in the fermentation broth is a critical step in the production process to ensure the quality and purity of the final product. This article discusses several methods for analysing HA concentration in fermentation broth. The description provides quantified details on the benefits and limitations of each method, thereby assisting in the selection of appropriate HA products for research or commercial purposes.
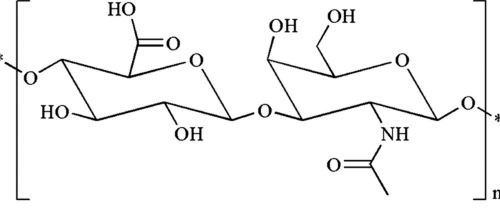
[1]
Figure 1. Chemical structure of HA
How is HA produced using fermentation broth?
Hyaluronic acid was traditionally extracted from rooster combs. This approach was abandoned due to a low extraction yield, a complex isolation process and high costs. Today, HA is produced on a large scale by microbial fermentation. HA is synthesised in living microorganisms by hyaluronan-synthase enzymes. This method offers the following advantages:
- New nutrients: Certain compounds are generated that facilitate absorption.
- Higher extraction rate: Proteases, cellulases and other enzymes break down plant cells, thereby releasing active compounds and increasing the yield of HA.
- Easier absorption: Polypeptides, polysaccharides and other small molecules in nanosize are produced, which can be absorbed more easily.
- Fewer side effects: Microbial cells degrade harmful substances, thereby reducing unsafe factors.
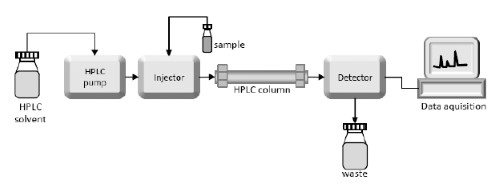
[2]
Figure 2. Diagram of HPLC systems
Further reading: Does fermented skincare work?
How is the HA concentration in fermentation broth analysed?
Numerous techniques have been employed to improve the efficiency, productivity and purity of hyaluronic acid production. Several approaches exist for analysing the HA concentration in the fermentation process.
- Precipitation with cetylpyridinium chloride
The precipitation with cetylpyridinium chloride (CPC) is a traditional analytical method. It is followed by spectrophotometric measurement at 540 nm. However, this method suffers from interference by other polysaccharides and exhibits low accuracy.
To overcome the limitations of the CPC method, alternative analytical techniques have been developed in recent years. In high-performance liquid chromatography (HPLC), HA molecules are separated based on their molecular weight and subsequently detected using a UV detector. HPLC is employed for its high sensitivity, accuracy and selectivity. It enables both the determination of molecular weight and the quantification of HA in the fermentation broth.
Capillary electrophoresis (CE) is a high-resolution technique that separates HA molecules according to their charge-to-size ratio. It is capable of determining both the molecular weight and the concentration of HA in the fermentation broth. CE requires low sample volumes and offers a short analysis time.
- Infrared spectroscopy
Recent efforts have focused on developing new analytical methods that address the limitations of existing techniques. A method based on infrared spectroscopy (IR) has been developed. In this method, the absorption spectra of HA molecules are measured directly and compared with a calibration curve, thereby determining the HA concentration. The method offers high sensitivity, low cost and simple sample preparation.
- Other methods
Other methods have also been developed. For example, mass spectrometry (MS) may be used to measure the molecular weight and concentration of HA by detecting ions produced by HA molecules. Gel permeation chromatography (GPC) can be employed to separate HA molecules by molecular weight, after which a spectrophotometric measurement is carried out at 205 nm.
Table 1 lists the advantages and disadvantages of these methods. For further information, please refer to the table below.
Table 1. Comparison of various analytical methods for HA concentration
|
|
Method |
Detection |
Advantages |
Disadvantages |
|
Precipitation with cetylpyridinium chloride |
Precipitation with cetylpyridinium chloride; |
Spectrophotometric measurement at 540 nm; |
Simple procedure; fewer reagents and minimal equipment required; |
Interference from other polysaccharides; Low accuracy; |
|
High-performance liquid chromatography |
Separation of HA molecules based on their molecular weight; |
Use of a UV detector; |
High sensitivity, accuracy and selectivity; |
Requires expensive equipment and trained personnel; Time-consuming; |
|
Capillary electrophoresis |
Separation of HA molecules based on charge-to-size ratio; |
Use of a UV detector and accompanying optics; |
High resolution; low sample consumption; short analysis time; |
Requires specialised equipment and expertise; Sensitive to electrode fouling; |
|
Infrared spectroscopy |
Direct measurement of the absorption spectra of HA molecules; |
Use of an IR spectrometer. |
High sensitivity; low cost; simple sample preparation; |
Less accurate; Certain contaminants may interfere with the measurement. |
Conclusion
The analysis of HA concentration in the fermentation broth is significant for the production of hyaluronic acid. Various analytical methods have been developed, and each exhibits its own limitations and benefits. The selection of a method depends on factors such as the sample matrix, the required sensitivity and accuracy, and the available equipment and resources. Development of new analytical methods is essential to improve the accuracy, sensitivity and cost-efficiency of HA analysis in fermentation broth.
Stanford Advanced Materials (SAM) is a reliable supplier of high quality hyaluronic acid in different grades and molecular weights. Please submit an enquiry if you are interested.
References:
[1] Wang, Yan & Cai, Li-Quan & Nugraha, Bramasta & Gao, Yi & Leo, Hwa. (2013). Current Hydrogel Solutions for Repairing and Regeneration of Complex Tissues. Current medicinal chemistry. 21. 10.2174/0929867321666131212151855.
[2] Czaplicki, Sylwester. (2013). Chromatographie in der Bioaktivitätsanalyse von Verbindungen. 10.5772/55620.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators





 Chin Trento
Chin Trento



