From Structure To Application: Is BIBO Or BBO The Better Crystal?
1 Summary
Beta-Barium-Borate (BBO) and Bismuth Triborate (BIBO) crystals are frequency‐doubling materials. They exhibit high transparency in the visible and near infra‐red regions and possess nonlinear optical properties that have led to their extensive use in nonlinear optical applications. Given the different nonlinear optical coefficients, BBO and BIBO are applied in distinct scenarios.
BBO has a higher nonlinear optical coefficient. In applications such as optical frequency doubling, summation, and differential frequency generation, its high coefficient increases conversion efficiency and produces stronger output signals at equivalent input power, thereby lowering device power requirements.
BIBO offers a moderate nonlinear optical coefficient which reduces optical losses and prevents saturation effects. Its coefficient remains relatively constant over a set temperature range. Consequently, it is employed in optical modulators, laser frequency doublers, and optical measurement systems.
This article compares BBO and BIBO crystals with regard to crystal structure, optical properties, application scenarios, production methods and costs, thereby providing a reference for selection.
2 Introduction to BBO and BIBO
Barium borate, known chemically as BaB₂O₄ or Ba(BO₂)₂, is an inorganic compound. It is available in both hydrated and anhydrous forms and appears as a white powder or colourless crystals. The crystals exist in two phases: the high‐temperature α-phase and the low‐temperature β-phase. Both exhibit birefringence, which makes the β-phase (BBO) widely suitable for nonlinear optical use.
Bismuth triborate (BiB₃O₆, BIBO) is a recently developed nonlinear optical crystal. It exhibits a high effective nonlinear optical coefficient, a high damage threshold and is not prone to deliquescence. It generally appears as colourless crystals.

3 Crystal Structure of BBO and BIBO
BBO belongs to the trigonal crystal system, whereby the borate ions are arranged in triangular forms and the barium ions fill the voids. BIBO belongs to the monoclinic system. Table 1 compares their chemical and structural properties.
Table 1: Chemical and Structural Properties
|
Crystal Structure |
Trigonal system Space group R3c |
Monoclinic system |
|
Unit Cell Parameters |
a = b = 12.532 Å |
a = 7.116 Å, b = 4.993 Å, |
|
Melting Point |
~1095 °C |
726 °C |
|
Mohs Hardness |
4 Mohs |
5–5.5 Mohs |
|
Density |
3.85 g/cm³ |
5.033 g/cm³ |
|
Thermal Expansion Coefficient |
α11 = 4×10⁻⁶/K |
αa = 4.8×10⁻⁵/K |
The crystals are classified as optically homogeneous (isotropic) or optically heterogeneous (anisotropic). The trigonal arrangement of BBO produces a uniaxial crystal with uniform properties along the a- and b-axes, whereas the monoclinic structure yields a biaxial crystal with distinct properties along each axis. Both crystals display nonlinear optical behaviour due to anisotropy.
4 Optical Properties of BBO and BIBO
4.1 Nonlinear Optical Properties of BBO and BIBO
The lack of central symmetry in these crystals precludes compliance with central symmetry conditions. As a result, atoms or molecules respond nonlinearly to the incident light field. The polarization rate is affected by variations in light intensity. Consequently, both BBO and BIBO exhibit high nonlinear optical coefficients that facilitate their specific applications.
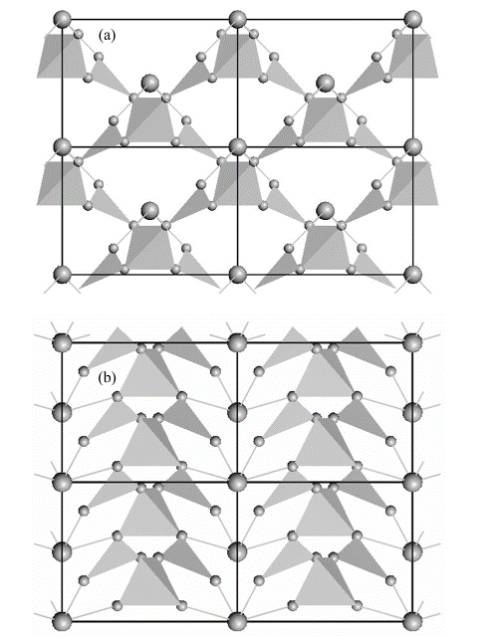
Note: (a): Projection of the cell along the c-axis; (b): Projection of the cell along the a-axis. The tetrahedra, triangles and atoms (Bi and O) are indicated [1].
BBO and BIBO differ with respect to optical properties. BBO has a higher nonlinear optical coefficient over a broader spectrum. Its coefficient is beneficial in optical frequency doubling, summation and differential frequency generation. BIBO possesses a slightly lower coefficient that may be advantageous in a particular wavelength range. In terms of transparency, BBO shows excellent performance in the visible and near infra-red; BIBO is marginally less transparent in the visible range.
BIBO crystals also exhibit low optical losses and maintain a broad transmission spectrum. Their nonlinear coefficient shows minimal variation with temperature over a defined range.
Table 2: Optical and Nonlinear Optical Properties
|
Transmission Range |
190–3500 nm |
286–2500 nm |
|
|
Absorption Coefficient |
<0.1%/cm at 1064 nm |
<0.1%/cm at 1064 nm |
|
|
1064/532 nm |
Ratio |
2.7 pm/V |
3.0 ± 0.1 pm/V |
|
Acceptance Angle |
0.8 mrad-cm (Type I, 1064 SHG) |
2.32 mrad-cm |
|
|
Departure Angle |
2.7° (Type I, 1064 SHG) |
25.6 mrad |
|
|
Temperature Bandwidth |
55 °C-cm |
2.17 °C-cm |
|
|
Sellmeier Equations |
nₒ² = 2.7359 + 0.01878/(λ² – 0.01822) – 0.01354λ² |
n₁²(λ) = 3.6545 + 0.0511/(λ² – 0.0371) – 0.0226λ² |
|
4.2 Introduction to Nonlinear Optical Coefficients
The nonlinear optical coefficient quantifies how a material responds to variations in light intensity. The response is nonlinear and depends on material properties, crystal symmetry and molecular structure. Light frequency and intensity also affect the nonlinear response, whereby increased frequency or intensity induces a higher nonlinear effect.
4.3 Factors Influencing the Nonlinear Optical Coefficients
The magnitude of the nonlinear coefficient directly impacts device performance. A higher coefficient in frequency-conversion applications increases conversion efficiency. In optical modulators, it influences both modulation depth and response speed.
5 Application Scenarios for BBO and BIBO
5.1 Advances in Optical Research
BBO exhibits a higher nonlinear optical coefficient than BIBO. Therefore, it is advantageous in certain frequency-conversion applications. In optical frequency doubling, summation and differential frequency generation, the higher coefficient produces a stronger output at constant input power and reduces energy requirements.
In optical research, Stanton EJ et al. [2] demonstrated Cherenkov phase matching at a bonded interface comprising nonlinear SiN and BBO crystals. The study quantified emission angle, conversion efficiency and output power via measurements of waveguide dimensions and pump power. The investigation thereby provides data for the production of compact devices for applications such as disinfection, free-space communications without line-of-sight and deep ultraviolet Raman spectroscopy.
Challenges and Limitations
Higher nonlinear coefficients may lead to increased optical losses. These losses lower device efficiency. In certain cases, saturation effects restrict the dynamic range and overall performance. Materials with elevated coefficients may also present reduced stability and durability. In these scenarios, BIBO is more suitable because its moderate coefficient and stable temperature behaviour help mitigate optical losses and extend the useful dynamic range.
6 Production Methods for BBO and BIBO
6.1 Production Method for BBO
BBO is produced by mixing Ba(OH)₂·8H₂O and H₃BO₃ in a molar ratio of 2:3. A flux is added during the reaction. After completion, the mixture is dried at 200–250 °C and then sintered at 500–600 °C for 4–5 hours to form low-temperature phase crystals. This low-temperature solid-state reaction method uses barium hydroxide and boric acid without additional complex steps. The method improves convection beneath the crystal, thereby reducing defects.
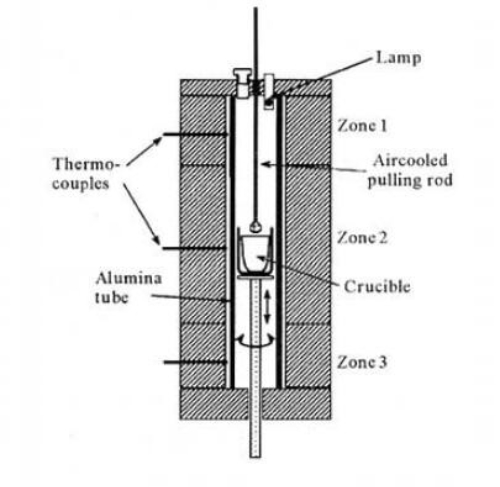
6.2 Production Method for BIBO (TSSG)
BIBO is grown using the top-seeded solution growth (TSSG) method. The melt is highly viscous, akin to glass-forming solutions. Equivalent stoichiometric quantities of Bi₂O₃ and B₂O₃ are ground thoroughly and melted in a platinum crucible at a constant temperature of 900 °C. A platinum wire is used to induce crystal growth, whereby spontaneous nucleation near a cooler section produces polycrystals that serve as seed crystals.
Because B₂O₃ has a much lower density than Bi₂O₃, it collects at the surface of the melt. This causes incomplete reaction and may yield Bi₂B₈O₁₅. Forced seed crystal growth is then applied, whereby transparent Bi₂B₈O₁₅ is chosen to seed the growth of polycrystals of BiB₃O₆ with a small amount of Bi₂B₈O₁₅ below the saturation point. The selection of BiB₃O₆ eliminates secondary growth and yields single crystals. Directional growth is required to obtain large, defect-reduced crystals with high single-crystal utilisation.
During growth, the seed crystal rotates at 3–5 r/min. The cooling rate is maintained at 0.1–1 °C per day, and the total cooling does not exceed 3–4 °C to prevent the formation of parasitic crystals. Finally, the crystal is withdrawn from the melt and cooled at 15–25 °C per hour until room temperature is reached. The cooling rate must not be too slow in order to prevent rapid glass formation and overrun by the expanding melt.
Conclusion
BBO and BIBO possess nonlinear optical properties that make them suitable for applications in lasers, electro-optical devices and other light-conversion equipment. BBO’s higher nonlinear optical coefficient improves the output-to-input power ratio and extends its application range. BIBO’s moderate coefficient, together with its stable temperature behaviour, effectively limits optical losses and maintains a wider dynamic performance range.
In production, the top-seeded method is used for BIBO. The production process for BBO is simpler and requires less stringent processing conditions than that for BIBO. The selection should be based on the application scenario, operational efficiency, stability and overall cost. For further guidance, please consult the SAM experts.
Further Reading:
Other Articles in Optics:
Ytterbium-doped Yttrium Aluminium Garnet
References:
[1] Zi-fang J, Jing-lin Y, Patrick S, et al. (2012) Microstructure study on bismuth triborate crystal and its melt at high temperature by Raman spectroscopy. Guang pu xue yu guang pu fen xi = Guang pu, 32(1).
[2] Stanton EJ, Tønning P, Ulsig EZ, Calmar S, Stanton MA, Thomsen ST, Gravesen KB, Johansen P, Volet N. (08/02/2024) Continuous-wave second-harmonic generation in the far-UVC pumped by a blue laser diode. Sci Rep, 14(1):3238. doi: 10.1038/s41598-024-53144-7; PMID: 38331948; PMCID: PMC10853522.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators




 Chin Trento
Chin Trento


