LaB6 Cathodes Used In Electron Microscopes
Introduction
Lanthanum hexaboride (LaB6, lanthanum boride or LaB) is a chemical compound composed of lanthanum and boron, and the lanthanum hexaboride target is a type of ceramic sputter target manufactured from the same material. Both are frequently used in optical instruments and techniques, for example in electron microscopes, electron lithography and other related fields. This article describes the use of lanthanum boride cathodes and lanthanum boride coatings in electron microscopes based on a successful example.

Figure 1 Lanthanum Hexaboride Sputter Targets
Features and Applications of LaB6 Sputter Targets
-Properties
Lanthanum hexaboride sputter targets display characteristics comparable to those of LaB6 ceramics. The purple-violet ceramic has a high melting point of 2 483 K. LaB6 is insoluble in water and hydrochloric acid. With a Mohs hardness of 9.5, it is mechanically strong. Given its low work function of approximately 2.70 eV and its capacity to emit electrons effectively, LaB6 is capable of generating high currents at lower temperatures. The material also exhibits a low evaporation rate.
-Applications
Owing to these technical properties, lanthanum hexaboride is used for cathodes in electron microscopes, scanning electron microscopes (SEM) and in electron beam lithography. High-purity LaB6 sputter targets may also be used to produce LaB6 thin-film coatings. This hexaboride is used in other fields such as aerospace, electronics and X‑ray powder diffractometry, and it is predominantly employed as a cathode material in electron microscopes.
Further reading: Properties and Applications of LaB6 Filaments
LaB6 Cathodes Compared with Tungsten Cathodes
-What are LaB6 Cathodes?
Electron sources are critical for the operation of electron microscopes. The performance of these instruments depends on a detector system. The optical arrangement comprises a cathode and one or two anodes with significant spacing between them. The complete configuration of a detector system is shown in Figure 2.
- The cathode functions as the electron source. Lanthanum hexaboride is an appropriate option for the production of such cathodes. Cathodes may also be coated with LaB6 thin films.
- The anode is arranged to attract and guide the electrons.
- Electrons are emitted from the solid surface of the cathodes once they acquire sufficient energy through heating. Consequently, the electrons are accelerated.
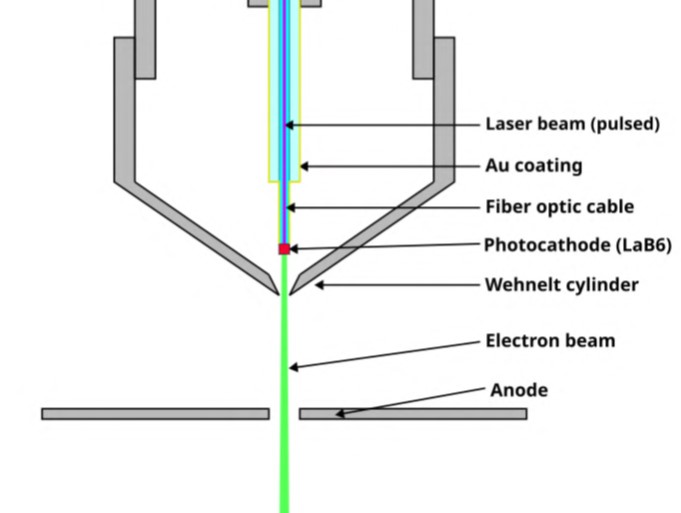
Figure 2 Structure of Lanthanum Hexaboride Cathodes
--Comparison of LaB6 Cathodes and Tungsten Cathodes
Various materials such as lanthanum hexaboride, cerium hexaboride and tungsten are employed in the manufacture of cathodes or cathode coatings to achieve enhanced electron emission. LaB6 offers the following advantages when compared with other cathode materials.
Tungsten:
- Tungsten has a shorter operational life due to evaporation and eventual structural breakdown.
- Tungsten cathodes are not usually recommended for optical instruments. Under high-temperature conditions, they may yield lower brightness and reduced image quality.
Lanthanum Hexaboride:
- LaB6 cathodes have an extended operational life, given that they are less prone to evaporation.
- LaB6 produces higher brightness because lower temperatures are sufficient for electron emission. These hexaboride cathodes are approximately 10 times "bright" compared with tungsten cathodes.
Lanthanum hexaboride shows marked advantages over tungsten as a cathode material. Its only drawback is a higher cost, which does not prevent its adoption as a cathode material for electron microscopes.
Case Study: LaB6 Cathodes in Electron Microscopes
--The Challenge
Stanford Advanced Materials (SAM) has supplied high-quality materials to laboratories for several years. Recently, a UK research customer requested several lanthanum hexaboride sputter targets for optical projects. The customer is a researcher who specialises in the fabrication of customised optical components such as laser devices and electron microscopes. The researcher aimed to build a detector system that could measure both cathodoluminescence and photoluminescence (see Figure 3). Consequently, the decision was made to utilise lanthanum hexaboride as the electron source rather than tungsten.
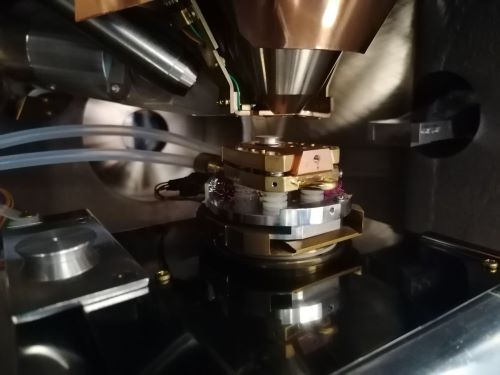
Figure 3 The Detector System
--The Solution
SAM is pleased to support its customers in the execution of their scientific studies. We also welcome learning further details about these projects and the optical instruments involved.
On this occasion, the customer provided details of his projects and requested a piece of LaB6 sputter target. The specified dimensions were 1" in diameter and 2–3 mm in thickness, with a purity of 99.5 %. He also attached an image of the electron microscope employed in his project (see Figure 4). Consequently, his experiments progressed satisfactorily with the LaB6 target supplied.

Figure 4 The Customer's Scanning Electron Microscope
--The Results
The customer acquired the lanthanum hexaboride material and observed additional advantages from the sputter targets. The researcher reported the following:
- Owing to the low work function of LaB6, a less expensive laser was adequate, as other coating materials require multiple non-linear processes.
- Compared with other materials that have a low work function, the degradation of the LaB6 coating was limited, thereby enabling simpler handling and conversion.
Conclusion
Lanthanum hexaboride presents several advantages when used for the fabrication of cathodes or cathode coatings in electron microscopes. Stanford Advanced Materials is a leading supplier of various ceramics, chemicals, magnets, metals and alloys. Please submit an enquiry if you are interested in lanthanum hexaboride or other modern materials. You may also visit our homepage for further details.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators





 Chin Trento
Chin Trento


