Main Applications Of Yttrium In Alloys And Phosphors
Introduction
Yttrium (chemical formula Y) is classified as a rare earth element. It exhibits physical and chemical properties comparable to those of other rare earth elements. Its classification is based on historical reasons. It is a soft, silver transition metal. It belongs to the lanthanide group. It is especially associated with the heavy rare earth group, which comprises elements with atomic numbers from 63 to 71. Yttrium’s electron configuration is [Kr]5s24d1. It tends to lose three electrons to obtain a stable structure with eight electrons. Consequently, its oxidation state is +3. Y2O3 is one of the most commonly used yttrium compounds.
Yttrium occurs in the Earth’s crust at 31 ppm. It is the 28th most abundant element and is approximately 26 000 times more common than gold (Au). It is typically obtained as a by-product alongside other lanthanides in rare earth minerals. The majority of yttrium is sourced from the following three materials:
- Xenotime: a phosphate mineral that contains yttrium orthophosphate (YPO4).
- Monazite: a reddish-brown phosphate mineral that contains rare earth metals.
- Bastnaesit: a calcium fluoro-carbonate mineral that contains cerium, lanthanum and yttrium.
Yttrium is used in many areas. It is applied as a phosphor in colour televisions, energy-efficient lighting and fuel cells [2]. It is also used in metallurgy, ceramics, superconductors and other fields. This article focuses on yttrium used in alloys and phosphors.
Yttrium as an Alloying Addition
Yttrium is added during alloy processing because it has a deoxidising, desulphurising, denitrogenating or degassing effect. This effect is attributed to its low thermodynamic oxidation potential [1]. The addition of a measured amount of yttrium to a Ni-20Cr alloy can improve its resistance to high-temperature oxidation considerably. The exact mechanism remains undetermined despite several hypotheses and research studies. Two explanations are discussed:
- The addition of yttrium can reduce the overall mass increase of the alloy. (Mass increase is the total weight gain due to the absorption of atoms or molecules from the environment. It may be caused by corrosion, oxidation and precipitation.)
- The addition of yttrium improves the surface adherence of the alloys.
An aluminium oxide alloy with yttrium addition is presented as an example.
Fe-20Cr-4Al Alloy and Yttrium Implantation
Fe-20Cr-4Al is an alloy composed of 20% chromium, 4% aluminium and the balance iron. It is used in high-temperature applications such as combustion chambers or heat exchangers. It exhibits good resistance to oxidation and corrosion at high temperatures.
The following procedure describes how yttrium is implanted into the Fe-20Cr-4Al alloy:
The Fe-20Cr-4Al alloy is repeatedly hot and cold rolled to produce a 0.5 mm thick sheet. The implanter is then used to implant yttrium ions into the alloy. Rutherford Backscattering Spectroscopy (RBS) is applied to measure the yttrium concentration accurately. In this case, yttrium is implanted at concentrations from 0.01% to 0.5%.
Experiment and Discussion of Results
Fe-20Cr-4Al alloys with 0, 0.01, 0.02, 0.05, 0.1, 0.2 and 0.5% yttrium were exposed to O2 at 1200℃ for 5 hours. Figure 1 shows a decrease in the mass gain for the Fe-20Cr-4Al alloy up to Fe-20Cr-4Al-0.1Y. Thereafter, the mass gain increases again [1].
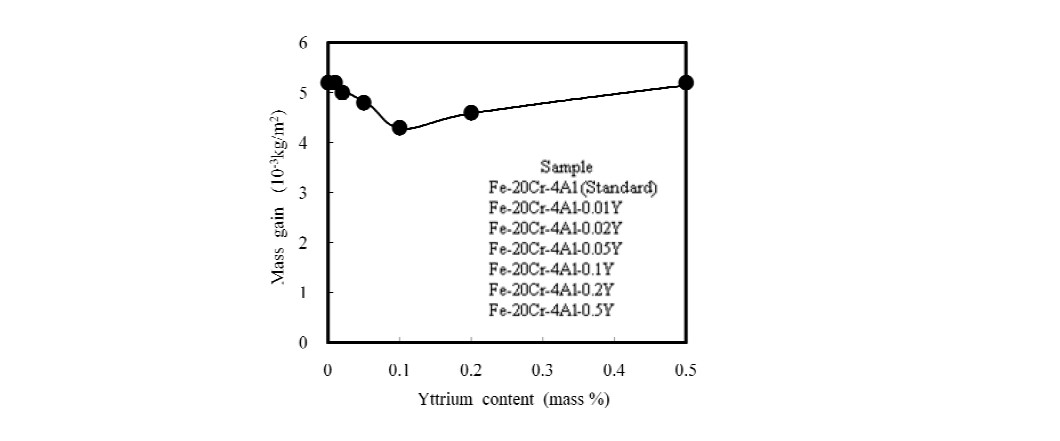
Figure 1: Change in the mass gain of Fe-20Cr-4Al-(0, 0.01, 0.02, 0.05, 0.1, 0.2, 0.3)Y alloys after 5 hours at 1200℃ in O2 [1].
Figure 2 shows the surface appearance of these alloys after exposure to O2. The surface of the standard FeCrAl alloy begins to delaminate. From images (b) to (h) an oxide surface forms to protect the material beneath. Images (b) and (c) show minor surface spallation. With increasing yttrium concentration, a more complete oxide surface develops. Alloys with 0.1Y to 0.5Y display darker surfaces than those with 0Y to 0.05Y. X-ray diffraction analysis of the alloy surfaces revealed the following observations [1]:
(a) A very weak crystalline Al2O3 surface structure is formed. From images (b) to (h) a strong crystalline Al2O3 layer is observed.
(b) Between images (f) and (h), a very weak crystalline Y3Al5O12 structure is observed.
Y3Al5O12, also known as yttrium oxide garnet (YAG), is a synthetic material. It displays high temperature resistance, high strength and chemical stability. YAG formation may explain the increased mass gain observed from 0.1Y to 0.5Y. This increase does not indicate a reduction in high-temperature oxidation resistance. In fact, the alloy exhibits better resistance to oxidation and corrosion at elevated temperatures with higher yttrium concentrations.
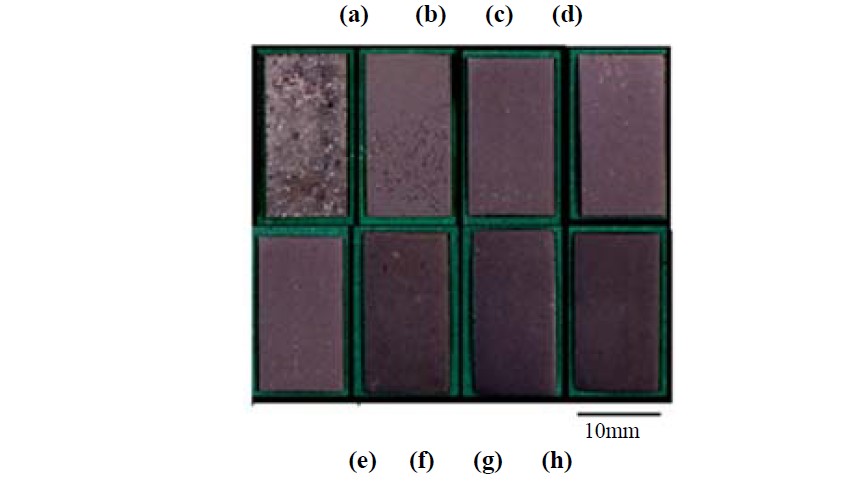
Figure 2: Surface photographs of FeCrAl-(0, 0.01, 0.02, 0.05, 0.1, 0.2, 0.3)Y alloys after 5 hours at 1200℃ in O2 [1]. (a) 0Y; (b) 0Y post cleaning; (c) 0.01Y; (d) 0.02Y; (e) 0.05Y; (f) 0.1Y; (g) 0.2Y; (h) 0.3Y
Use of Yttrium in Phosphors
Phosphors are substances that absorb radiation and emit light. Their function relies on orbital electrons absorbing radiation energy, becoming excited to higher energy levels and then returning to their ground state. The energy released during this process produces light.
The elements incorporated in phosphors directly affect the emitted light. Due to its stable, narrow and efficient red emission, Y2O3 is used in phosphors for colour televisions, computer monitors, light-emitting diodes (LEDs) and X-ray intensifier screens [2].
Standard LEDs produce a cold white light. Phosphor-converted warm white LEDs (pc-WLEDs) represent the new LED technology [3]. Nano-sized Y2O3 can be added to the phosphor to introduce red components, thereby providing a warmer and qualitatively enhanced light.
Conclusion
Yttrium is one of the rare earth elements. Owing to its distinct properties, it is employed in both phosphors and alloys. Many applications and yttrium compounds remain unmentioned. Stanford Advanced Materials (SAM) offers various types of yttrium. For further information regarding yttrium or its compounds, please contact our technical staff with your application details.
References
- Volkerts, B. D. (Ed.). (2010). Yttrium: Compounds, production, and applications: Verbindungen, Herstellung und Anwendungen. Nova Science Publishers, Incorporated.
- Zhang, K., Kleit, A. N., & Nieto, A. (2017). An economic strategy for criticality – application to the rare earth element yttrium in the new lighting technology and its sustainable availability. Renewable and Sustainable Energy Reviews, 77, 899–915. https://doi.org/10.1016/j.rser.2016.12.127
- Petry, J., Komban, R., Gimmler, C., & Weller, H. (2022). Simple one-pot synthesis of luminescent europium doped yttrium oxide Y2O3:Eu nanodiscs for phosphor-converted warm white LEDs. Nanoscale Advances, 4(3), 858–864. https://doi.org/10.1039/d1na00831e

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators





 Chin Trento
Chin Trento



