Navigating The World Of Membrane Filters: Types, Uses, And Benefits (Ⅰ)
Introduction
Membrane filtration technology is a physical method that uses thin or porous membranes to separate, filter and purify small particles from liquids or gases. The membranes are typically fabricated from synthetic materials (e.g. polyester, polyamide, polycarbonate, etc.) and retain solid particles, microorganisms, dissolved substances or solvents, thereby purifying the fluid. The pore sizes may range from nanometres to micrometres depending on the filtration target. In contrast to conventional separation methods such as precipitation, adsorption or ion exchange, membrane filtration offers simplicity in operation, high filtration precision and low energy consumption. This article presents the properties, manufacturing processes and application areas of various membrane filters based on the materials from which they are produced, thereby providing a reference for selection.
2 What are Membrane Filters?
Membrane filters are frequently employed in laboratories, industrial production, water treatment, food processing, pharmaceuticals and other fields to separate and purify liquids or gases. They filter fluids by utilising membranes with specific pore sizes or predefined specifications so that particles of a defined size or type are separated.
Membrane filters operate on the principle of size exclusion whereby particles are selectively blocked according to their size relative to the membrane pores. Consequently, they can be categorised according to the pore size of the membrane. For example, microporous membranes can remove most microorganisms, bacteria and macromolecular dissolved substances, whereas ultrafiltration membranes isolate larger particles such as proteins and colloids.
Membrane filters may also be classified by the type of material used in their fabrication. The most common types are generally based on polymers (such as polyethersulphone (PES), polyvinylidene fluoride (PVDF), etc.), ceramics (e.g. zirconium dioxide and alumina-based ceramics), nanostructures (including titanium dioxide nanotubes, graphene oxide (GO) and carbon nanotubes (CNT)) and metal-organic frameworks (MOFs). These filtration membranes have different properties and are used in various areas of production and research.
Due to space limitations, this article concentrates on polymer and ceramic membranes. Further details will be provided on our website in a later Part II, which discusses the advanced applications and properties of nanostructured membranes and metal-organic frameworks.
Fig. 1 Membrane Filters for Water Purification
3 Polymer Membranes
3.1 Polyethersulphone (PES)
3.1.1 Introduction to Polyethersulphone
Polyethersulphone (PES) is an engineering plastic with defined chemical and physical properties. Its chemical structure comprises repeating phenyl-ether-sulphone units that form a linear or branched polymer chain. The benzene rings and ether bonds contribute to thermal stability and mechanical strength. Sulphone groups increase heat resistance and chemical stability. The glass transition temperature is 225 ℃ and the material can be used for prolonged durations at 180 ℃. PES materials exhibit resistance to oxidation, corrosion and are flame retardant, in addition to possessing acceptable blood compatibility and overall performance.
3.1.2 How to Produce Polyethersulphone
1. Phase-Inversion Method: The phase-inversion method is straightforward, cost-effective and widely applied. The membrane formation process involves formulating a homogenous polymer solution of a specific composition. Physical methods induce solvent and non-solvent mass transfer, thereby changing the thermodynamic state of the solution. This results in separation from the homogenous phase and conversion into a three-dimensional macromolecular network gel structure. The membrane subsequently cures. The film formation methods include nonsolvent-induced phase separation (NIPS), thermally induced phase separation (TIPS) and vapour-induced phase separation (VIPS).
2. Electrospinning Method: In the electrospinning process, a fibrous membrane is produced that has a high specific surface area, high porosity, good connectivity, a small fibre diameter and controllable membrane thickness. The process involves placing the prepared spinning solution into a high-voltage electric field. Under this field, capillary Taylor cone droplets are accelerated to overcome surface tension, forming a jet of fine streams. The solvent evaporates from the jet, and the residue solidifies on the collecting substrate, resulting in a fibre mat. Compared with the phase-inversion method, electrospinning is easier to operate and has a higher production efficiency, thereby meeting the demands of various application scenarios.
3. Coating Method: Unlike the previous methods, the coating method applies a separation layer onto a base film (e.g. PSF or PES film). This additional layer plays a role in the separation performance of the composite membrane. The method is straightforward; however, it is necessary to ensure the uniformity and adhesion of the coating to maintain the stability and reliability of the filter membrane during use.
3.1.3 Applications of Polyethersulphone
1. Biomedical Applications: PES exhibits satisfactory biocompatibility and is used in the manufacture of blood purification materials, wound dressings and biological scaffolds for biomedical purposes. PES membranes are commonly utilised in the production of haemodialysis membranes due to their antifouling, antibacterial, anti-coagulation and biocompatible properties.
2. Water Treatment: PES membranes are used in water treatment for the production of purified water, oil–water separation, desalination and various forms of wastewater treatment. In practice, polydopamine (PDA) and polyethyleneimine (PEI), loaded with isolated iron species (4A-Fe) as catalyst particles, are applied to PES membranes. This configuration is capable of effectively separating non-emulsified oil–water mixtures and degrading phenolic pollutants. The separation efficiency is reported to reach 99.8%, thereby reducing phenol contamination.
3. Battery Applications: Owing to its mechanical properties and heat resistance, the PES membrane is used as a battery separator in lithium-ion batteries, methanol fuel cells and microbial fuel cells. Prior to electrospinning, PES is added to the polyvinylidene fluoride (PVDF) spinning solution to produce a heat-resistant composite PES/PVDF fibre membrane for lithium-ion battery diaphragms. The composite membrane exhibits an ionic conductivity of 1.69 × 10-3 S/cm.

Fig. 2 Polyethersulphone Membrane – Folded Cartridge
3.2 Polyvinylidene Fluoride (PVDF)
3.2.1 Understanding Polyvinylidene Fluoride (PVDF)
Polyvinylidene Fluoride (PVDF) is a synthetic polymer produced by the polymerisation of vinylidene fluoride (VDF) monomer. PVDF is a transparent, colourless thermoplastic with defined properties. It is resistant to acids, alkalis, organic solvents and other chemicals and maintains its stability at temperatures up to 150 °C. PVDF also demonstrates satisfactory weather resistance, enabling its use in outdoor environments over extended periods without adverse effects from ultraviolet light, oxidation or moisture. Its transparency allows both visible and ultraviolet light to pass through. Moreover, PVDF is biocompatible and is frequently employed in medical applications.

Fig. 3 Polyvinylidene Fluoride Membrane Filter
3.2.2 Synthesis Methods for Polyvinylidene Fluoride (PVDF)
1. Dry Polymerisation: In dry polymerisation, also known as gas-phase polymerisation, vinylidene fluoride (VDF) gas reacts with a catalyst, such as iron fluoride or iron(III) chloride, to produce a PVDF polymer. A benefit of this method is that no solvent is used, thereby reducing the need for solvent removal in subsequent processing steps. After polymerisation, a thermal treatment is typically applied to ensure complete crystallisation of the polymer and the removal of residual catalyst. This thermal process includes heating, cooling and crystallisation stages. The resulting polymer is then processed by extrusion or calendaring to form a PVDF film.
2. Wet Polymerisation: In this method, the vinylidene fluoride (VDF) monomer is dissolved in a suitable solvent such as hydrogen fluoride, trichloroethylene, or methylene chloride. The monomers undergo radical or anionic polymerisation in solution. An initiator, for instance a peroxide compound, is added to trigger the polymerisation. The initiator produces free radicals, thereby promoting chemical bonding between monomer molecules to gradually form polymer chains. The reaction is conducted under controlled temperature and pressure to manage polymer formation and molecular weight. Following the reaction, the solvent is removed by evaporation or vacuum processing, yielding the PVDF polymer as a solid, which is subsequently processed into a film.
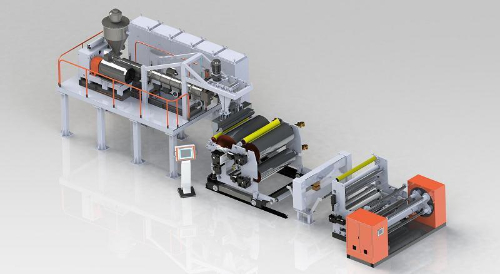
Fig. 4 Schematic Illustration of the Production Line for PVDF Membrane Filters
3.2.3 Applications of Polyvinylidene Fluoride (PVDF)
1. Removal of Microorganisms: The microporous structure of PVDF membrane filters effectively removes microorganisms and bacteria. They are used in applications such as drinking water treatment and in food and beverage production processes that require strict microbial control. The pore size and distribution can be adjusted by controlling process parameters and adding appropriate pore-forming agents during membrane production. Typically, these micropores range from nanometres to micrometres, thereby blocking most bacteria, viruses and parasitic eggs and enabling efficient filtration.
2. Separation and Purification of Chemicals: PVDF membrane filters are widely applied in the chemical industry for chemical separation and purification. Due to their chemical and solvent resistance, PVDF membranes facilitate the recovery and reuse of organic solvents. By filtering the reaction mixture through a PVDF membrane, solvents and reaction products can be effectively separated. The microporous structure of the membrane blocks larger molecules and particulates while allowing smaller dissolved molecules to pass through. This process clarifies the solution for subsequent purification stages.
3. Electronics Industry: In the electronics industry, PVDF membrane filters are used to remove particulate matter during the manufacture of electronic devices and to filter photoresists. In semiconductor production, controlling particulate contamination is critical. PVDF membranes filter particulates from air, solvents and process water, thereby reducing contamination in the production environment and lowering product defect rates. In photolithography, PVDF membranes remove impurities from photoresist solutions, ensuring clear lithographic patterns.
Table 1 Comparison of the Properties of PES and PVDF
|
Properties |
Polyethersulphone (PES) |
Polyvinylidene Fluoride (PVDF) |
|
Chemical Structure |
Repeating phenyl-ether-sulphone units Linear or branched polymer chains |
Polymerisation of vinylidene fluoride monomers |
|
Thermal Stability |
Glass transition temperature up to 225 °C Long-term use possible at 180 °C |
Stable at temperatures up to approximately 150 °C |
|
Physical Properties |
Resistance to oxidation and corrosion; flame retardant; satisfactory mechanical strength |
Chemical and weather resistance; high light transmittance; biocompatibility |
|
Manufacturing Methods |
Phase inversion Electrospinning Coating methods |
Dry polymerisation Wet polymerisation |
|
Application Areas |
Biomedical devices Water treatment Electronics manufacturing |
Microbial removal Chemical separation Electronics fabrication |
|
Advantages |
Resistance to oxidation; flame retardancy; biocompatibility |
Chemical resistance; resistance to weathering; light transmittance |
|
Disadvantages |
Manufacturing involves elaborate processes and higher costs |
Relatively high production costs; susceptible to photo-oxidation |
|
Applications |
Haemodialysis membranes Water purification Battery diaphragms |
Microbial filtration Chemical purification Electronics fabrication |
4 Ceramic Membranes
4.1 Overview of Ceramic Membranes
Ceramic filtration membranes are thin layers produced from ceramic materials for the filtration, separation and purification of liquids or gases. These membranes, typically fabricated from materials such as zirconium dioxide (ZrO2) or aluminium oxide (Al2O3), possess a microporous structure. The pore size and distribution can be controlled to achieve selective separation of particles or molecules based on size.
The ceramic membrane exhibits high temperature resistance, maintaining stability in high-temperature environments. In addition, many chemicals display good chemical stability, thereby resisting corrosion. Ceramic membranes also possess a certain level of mechanical strength and resistance to abrasion, enabling them to withstand pressures and loads encountered during operation. These characteristics underpin their use in scientific research and industrial production.
4.2 Structure of Ceramic Membranes
1. Carrier Material: Ceramic membranes typically require a supporting substrate to ensure mechanical stability and adhesion. The substrate may be made from metal, ceramic or other materials; common substrates include alumina, silicon and titanium. The compatibility and adhesion between the substrate and the membrane material are critical factors in the selection process.
2. Functional Layer: This is the primary layer of the ceramic membrane, usually composed of ceramic materials such as zirconium dioxide (ZrO2) or aluminium oxide (Al2O3). The thickness typically ranges from a few micrometres to several tens of micrometres depending on the application requirements. The microporous structure of this layer is central to achieving the filtration function, and its pore size and distribution can be controlled by adjusting the fabrication process.
3. Surface Modification: In order to improve the performance or adapt the membrane for a specific application, the surface of the functional layer may be treated. Such treatments may involve chemical modification, coating or other functional alterations to enhance selectivity, stability or biocompatibility.
4. Pore Structure: The effectiveness of the ceramic membrane relies primarily on its pore structure. The pores may be microporous, mesoporous or macroporous; their size and distribution determine the membrane’s filtration characteristics. Microporous structures are generally used for the separation of small molecules or particles, whereas macroporous structures are employed in high throughput filtration applications.
4.3 Processes in the Synthesis of Ceramic Membranes
Taking the example of a zirconium dioxide-based ceramic membrane: a dispersant such as polyethylene glycol or nitric acid is added to an inorganic zirconium salt solution, which reacts under heat. Oxalic acid is then introduced to produce an oxalic acid–oxo-zirconium sol. This sol undergoes a hydrothermal process to yield a zirconium dioxide nanoparticle suspension. The nanoparticles are then mixed with plasticisers and binders to form a coating solution. Subsequent coating, calcination and cooling steps yield the ceramic ultrafiltration membrane made from zirconium dioxide. The method produces nanoparticles of uniform size. The membrane layer exhibits minimal shrinkage and is less prone to cracking, thereby resulting in a defect‐free zirconium dioxide ceramic ultrafiltration membrane. The process is straightforward and cost effective.
4.4 Utilisation of Ceramic Membrane Performance in Applications
1. Food and Beverage Industry: In the food and beverage industry, ceramic filtration membranes are used to clarify and filter fruit juices, wines, beers and dairy products. They remove suspended solids such as pulp, skins, seeds and microorganisms, thereby enhancing product clarity and extending shelf life. In winemaking, ceramic membranes remove precipitates such as yeast, proteins and tartrate crystals, thereby improving the appearance and stability of the wine. In dairy processing, ceramic membranes clarify emulsions by removing proteins, milk fats and bacteria, thus contributing to improved purity, taste and longevity while ensuring product safety and hygiene.
Further Reading: Ceramic Membranes and their Application in Food and Beverage Processing

Fig. 5 Equipment for Beverage Purification with Ceramic Filtration Membranes
2. Water Treatment: Ceramic filtration membranes are employed in drinking water purification to remove suspended solids, microorganisms, organic compounds and other contaminants. These membranes enable effective particulate filtration, thereby assisting in meeting drinking water standards. In wastewater treatment, ceramic membranes are used for solid-liquid separation and the removal of contaminants and microorganisms, ensuring that the treated water conforms to discharge or reuse quality standards. Moreover, ceramic membranes are applied in pre-treatment, desalination and post-treatment processes to remove salts and other impurities from seawater. They are also utilised in industrial wastewater treatment, water resource reclamation and in improving the water quality of rivers and lakes, thereby contributing to environmental protection.
What to Expect in Part II
Following Part I of this series, which addressed traditional membrane filters such as polymer and ceramic membranes, Part II will examine advanced membrane technologies. In the forthcoming sections, the structure, synthesis and various applications of nanostructured membranes, composite filter membranes and other topics will be discussed. Please follow Stanford Advanced Materials (SAM ) for further insights into the evolving field of membrane filtration.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators




 Chin Trento
Chin Trento