A Comprehensive Guide To Amorphous Metals
1. Introduction to Amorphous Metals
Amorphous metals, also referred to as metallic glasses, are a class of materials characterised by a disordered atomic structure. In contrast to crystalline metals, which have a regular, repetitive atomic arrangement, amorphous metals lack this order. The absence of crystallinity yields high tensile strength, elasticity and corrosion resistance. These properties render the materials applicable for advanced applications.
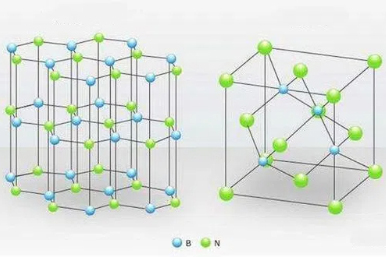
 [1]
[1]
2. Manufacturing Processes
Amorphous metals are generally produced via rapid cooling processes, thereby preventing atoms from arranging in a crystalline structure. Common methods include:
- Melt Spinning: Molten metal is rapidly cooled on a rotating wheel, resulting in thin ribbons. This method is widely used in the production of amorphous metal ribbons for transformers and other magnetic applications.
- Splat Quenching: A droplet of molten metal is rapidly cooled between two cold surfaces, thereby producing thin, flat sheets of amorphous metal. This method is employed in laboratory settings for rapid material analysis and small-scale production.
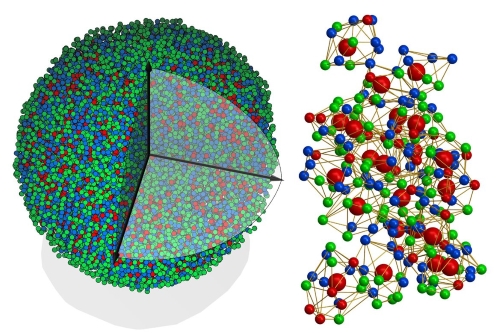
 [2]
[2]
- PVD (Physical Vapour Deposition): Metal atoms are deposited onto a substrate in a vacuum environment, thereby enabling the controlled formation of amorphous layers. This technique is frequently employed in the electronics industry for the production of thin layers with specific magnetic or optical properties.
3. Properties and Applications
Amorphous metals exhibit several quantifiable properties, including high tensile strength, elasticity and corrosion resistance:
- High tensile strength: Amorphous metals often demonstrate higher tensile strength than their crystalline counterparts due to the absence of grain boundaries. For example, metallic glass alloys such as Vitreloy 1 exhibit a tensile strength of up to 1.9 GPa, which is considerably higher than that of conventional steel.
- Elasticity: Such metals can experience elastic strains of up to 2%, whereas crystalline metals typically show elastic strains of approximately 0.2%.
- Corrosion resistance: The absence of grain boundaries and the homogeneous structure contribute to excellent corrosion resistance. For instance, zirconium-based metallic glasses have demonstrated high corrosion resistance in saline environments, thereby making them suitable for maritime applications.
- Magnetic properties: Certain amorphous metals display soft magnetic characteristics. Amorphous iron alloys, for example, present lower coercivity and reduced core losses compared to crystalline iron, thereby improving energy efficiency in transformers.
- Electrical resistance: Another quantifiable feature is the high electrical resistance, which can be advantageous in applications such as resistors and magnetic sensors.
The properties of amorphous metals have led to their use in various sectors:
- Electronics: They are used in transformer cores and inductors, particularly in high-frequency applications where low energy loss is essential. Amorphous metal cores reduce energy losses by up to 70% compared with conventional silicon steel cores.
- Biomedical devices: Owing to their biocompatibility and corrosion resistance, these materials are suitable for medical implants and surgical instruments. Zirconium-based metallic glasses are used in stents and orthopaedic implants.
- Sports equipment: They are employed in high-performance sports devices, such as golf clubs and tennis rackets. The elasticity of metallic glass contributes to improved energy transfer, thereby enhancing device performance.
- Defence and aerospace: They find use in light armour and structural components where a high strength-to-weight ratio is required. Amorphous metal coatings are also applied to protect aerospace components from wear and corrosion.
- Consumer electronics: They are employed in casings and structural components due to their durability and scratch resistance. For example, a metallic glass alloy is used in the casing of the Apple Watch, valued for its strength and smooth surface.
4. Challenges and Developments
Amorphous metals face several challenges that restrict their widespread adoption. The main obstacles include production costs, size limitations and brittleness, each of which imposes significant constraints in various contexts.
The primary challenge is the high production cost. The rapid cooling of molten metal, required to prevent crystallisation, necessitates specialised equipment and precise control. Consequently, the manufacturing process is complex and expensive. The need for rapid cooling typically requires the use of advanced machinery, thereby limiting large-scale production. As a result, their use is largely confined to applications where the benefits justify the production costs.
Another important limitation is the difficulty in producing large, bulk amorphous metal components. The rapid cooling necessary to retain the amorphous structure becomes increasingly challenging as component size increases. Consequently, most amorphous metals are available only in small forms such as ribbons, wires or thin sheets. This restriction confines their application to smaller items and niche markets.
Brittleness also remains a critical issue, particularly in structural applications where materials must withstand significant loads. Although amorphous metals are recognised for their strength, the lack of a crystalline structure may result in brittleness, thereby increasing the risk of fracture under certain conditions. This brittleness is especially problematic in applications that require materials to absorb shocks or deform without fracturing.
In response to these challenges, significant progress has been achieved in the field of amorphous metals:
- Bulk Metallic Glasses (BMGs): This approach involves the development of larger amorphous metal components for industrial use. For instance, BMGs with enhanced ductility have been developed, thereby making them more appropriate for structural applications in the automotive and aerospace sectors.
- Advanced alloys: New compositions have been developed that improve the properties of amorphous metals, for instance, by enhanced ductility or increased corrosion resistance. Metallic glasses based on Pd and Cu exhibit improved mechanical performance.
- Additive manufacturing: Research is underway into the utilisation of 3D printing techniques to produce complex amorphous metal structures. This approach could enable the production of custom components with well-defined properties, for example, dental implants and intricate parts for aerospace.
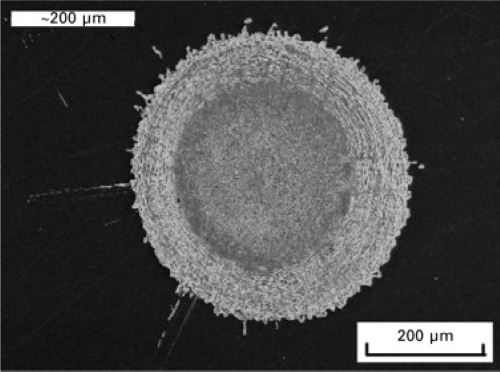
 [3]
[3]
5. Amorphous Metals vs. Metallic Glasses
The terms "amorphous metals" and "metallic glasses" are often used interchangeably. They refer to the same class of materials. However, subtle differences in their usage are important to understand.
--Amorphous Metals
Amorphous metals are metals with a disordered atomic structure that does not exhibit the regular, repeating pattern found in crystalline metals. This disordered structure is achieved by rapidly cooling the molten metal, thereby preventing the atoms from forming a crystalline lattice.
The term "amorphous metal" emphasises the atomic disorganisation and is commonly used when referring to the broader category, which includes various manufacturing processes and applications.
--Metallic Glasses
Metallic glasses are a subset of amorphous metals that specifically exhibit a glass-like structure. This term highlights the non-crystalline, glass-like state of the material that - while produced from metallic alloys - resembles conventional glasses such as quartz glass.
The term "metallic glass" is often employed in scientific and academic contexts, particularly when discussing the physical and mechanical properties associated with the glass-like state, such as brittleness and elastic behaviour.
In summary, although the terms "amorphous metals" and "metallic glasses" refer to the same general type of material, the former is a broader term that is more commonly used in industry, while the latter is more specific and frequently employed in scientific research to denote the glass-like properties of these materials. Understanding these distinctions aids in accurately describing the material properties and potential applications.
6. Conclusion
Amorphous metals, with their disordered atomic structure, represent an important development in materials science. Their combination of high tensile strength, elasticity and corrosion resistance distinguishes them from conventional crystalline metals and renders them essential in electronics, biomedical devices, defence and aerospace.
Despite the challenges arising from high production costs, limited component size and brittleness, research and innovation continue to expand the achievable scope with these materials. Given that industry seeks materials which meet the demands of modern technology, amorphous metals are positioned to influence the future of high-performance applications. Further information is available at Stanford Advanced Materials (SAM).
References:
[1] UCLA News (2021, 31/03/2021). Jahrhundertaltes Problem mit der ersten atomaren 3D-Darstellung eines amorphen Festkörpers gelöst. Retrieved on 20/08/2024, from https://newsroom.ucla.edu/releases/first-ever-3d-atomic-imaging-amorphous-solid
[2] Y.C. Xin, P.K. Chu, 11 - Plasma immersion ion implantation (PIII) of light alloys, Editor(s): Hanshan Dong, in Woodhead Publishing Series in Metals and Surface Engineering, Surface Engineering of Light Alloys, Woodhead Publishing, 2010, pages 362–397, https://www.sciencedirect.com/science/article/pii/B9781845695378500117
[3] Universität Wien (2024, 20/08/2024). Strukturelle Inhomogenitäten in massiven metallischen Gläsern. Retrieved on 20/08/2024, from https://sounds-of-matter.univie.ac.at/research-projects/metallic-glass/

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Chin Trento
Chin Trento