Substrates, Supports, And Ligands In Precious Metal Catalysts
Introduction
Precious metal catalysts are employed in numerous industrial applications for their catalytic properties. Their performance is significantly affected by the materials to which they are bound, that is, substrates, supports or ligands. These materials determine the catalyst’s activity, stability, selectivity and regenerability. The following text provides a detailed overview of these components and their significance for catalytic reactions.
1. The Substrate: The Basis of Catalytic Reactions
A substrate serves as a surface on which precious metals are deposited or dispersed during catalytic reactions. This is particularly important for heterogeneous catalysts. The choice of substrate is crucial because it affects the dispersion, surface area and catalytic activity of the precious metals.
Common support materials are:
- Alumina (Al₂O₃): Aluminium oxide is noted for its high surface area and adequate mechanical strength; consequently, it is used in hydrogenation, oxidation and reforming reactions.
- Silica (SiO₂): Silicon dioxide substrates are chemically inert and thermally stable. They are suited for catalytic processes that require high selectivity.
- Carbon materials: Activated carbon and carbon nanotubes provide high electrical conductivity and a high surface area. They are used for fuel cell catalysts and certain reduction reactions.
2. Supports: Enhancing Catalyst Performance
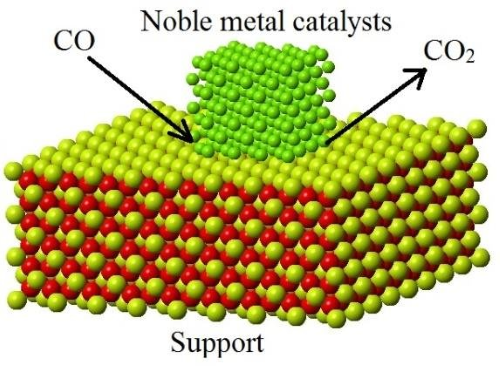 [1]
[1]
Supports are materials that facilitate the dispersion of precious metals over the catalyst surface. Their main function is to provide a high surface area for metal dispersion while maintaining the catalyst’s activity.
Porous Oxides
Porous oxides such as aluminium oxide, silicon dioxide and titanium dioxide are used because of their high surface area and thermal stability. They are appropriate for dispersing precious metals.
- Alumina (Al₂O₃): Widely used because of its surface area (100–300 m²/g) and stability in high‐temperature processes such as hydrogenation and reforming.
- Silica (SiO₂): Selected for its inertness and thermal stability (200–600 m²/g), it is suited for oxidation reactions and catalytic cracking.
- Titanium dioxide (TiO₂): Known for its photocatalytic properties, it is used in light‐activated processes and in emission control in the automotive industry.
Carbon Supports
Carbon supports, including soot and activated carbon, are essential for electrochemical applications due to their conductivity and high surface area.
- Soot: Provides high conductivity and is frequently used in fuel cells, where platinum on soot (Pt/C) plays a key role in oxygen reduction.
- Activated carbon: With its high surface area (500–1500 m²/g), it is used for adsorption and filtration processes, thereby assisting reactions such as hydrogenation.
Metal Oxides
Metal oxides such as ceria and zirconia offer distinct redox properties that enhance the interaction with precious metals and increase catalytic efficiency.
- Ceria (CeO₂): Effective in oxidation–reduction reactions, particularly in automotive catalytic converters due to its oxygen storage capacity.
- Zirconia (ZrO₂): Known for its thermal stability and durability under harsh conditions, it is used in high‐temperature isomerisation processes.
1. Ligands: Tuning Catalytic Properties
Ligands are molecules or ions that form coordination bonds with the precious metal centre. They are mainly used in homogeneous catalysts. The structure and properties of ligands directly affect the activity, selectivity and stability of the catalyst.
The following outlines common types of ligands:
- Phosphine ligands: Compounds such as Triphenylphosphine (PPh₃) are frequently employed in palladium-catalysed cross-coupling reactions to control the reaction rate and selectivity.
- Nitrogen-containing ligands: Ligands such as pyridine and bipyridine adjust the electronic density of precious metals, thereby affecting catalytic activity and selectivity.
- Chelating ligands: Ligands such as EDTA form stable chelates with precious metals, increasing catalyst stability in complex organic reactions.
Factors Affecting Catalyst Performance
The performance of precious metal catalysts is determined by several factors related to substrates, supports and ligands.
- Surface area and porosity: The surface area and porosity of substrates and supports affect the dispersion of precious metals and the availability of active sites.
- Chemical stability: The chemical stability of supports and ligands determines the catalyst’s durability under conditions such as high temperatures or strongly acidic/alkaline environments.
- Electronic effects and coordination environment: The electronic properties and coordination provided by the ligands significantly influence the reaction pathways and selectivity of the catalyst.
Tailored Catalysts for Specific Industrial Applications
The selection of substrate, support and ligand combinations is guided by the specific requirements of various industrial applications. These materials must be chosen carefully to match the reaction conditions and the desired outcomes.
For example:
- Hydrogenation reactions: Catalysts on alumina supports are frequently used because of their high surface area and mechanical strength in hydrogenation processes.
- Fuel cells: Precious metal catalysts on carbon supports are indispensable in fuel cells, where high conductivity and chemical stability are required.
- Pharmaceutical synthesis: Ligand-modified catalysts are employed in pharmaceutical synthesis to achieve high selectivity and efficiency in complex organic reactions.
|
Application |
Catalyst Component |
Key Materials |
|
Hydrogenation Reactions |
Substrate |
Alumina (Al₂O₃) |
|
Substrate |
Silica (SiO₂) |
|
|
Fuel Cells |
Support |
Black Carbon (Pt/C) |
|
Support |
Graphene |
|
|
Pharmaceutical Synthesis |
Ligand |
Phosphine-modified Palladium (Pd/PPh₃) |
|
Ligand |
Chiral Ligands (e.g., BINAP) |
|
|
Oxidation Reactions |
Support |
Ceria (CeO₂) |
|
Substrate |
Titanium dioxide (TiO₂) |
|
|
Reforming and |
Support |
Zirconium dioxide (ZrO₂) |
|
Substrate |
Alumina (Al₂O₃) |
|
|
Polymerisation |
Support |
Ziegler-Natta (TiCl₄/MgCl₂) |
|
Support |
Metallocene (supported on Silica/Alumina) |
Additional cases and examples are available at Stanford Advanced Materials (SAM).
Further reading: Common Reaction Types of Homogeneous Precious Metal Catalysts
Conclusion:
The selection of appropriate substrates, supports and ligands is crucial for optimising the performance of precious metal catalysts. By carefully choosing these materials, the catalyst properties can be tailored to the specific requirements of various industrial applications, thereby increasing efficiency and extending catalyst lifespan.
Reference:
[1] Hossain, Shaikh. (2018). Synthesis and Kinetic Study of CeO₂ and SiO₂ Supported CuO Catalysts for CO Oxidation. 10.13140/RG.2.2.31499.80165.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Chin Trento
Chin Trento