Chlorine Trifluoride For In Situ Cleaning Of CVD Chambers In Semiconductor Manufacturing: Cons And Pros
Introduction
In the semiconductor industry, cleaning gases are used for the in-situ cleaning of CVD chambers. Chlorine trifluoride (ClF3) is one cleaning gas described on the Wikipedia page. ClF3 removes residues effectively while presenting measurable challenges because of its reactivity and corrosivity. This article examines the quantifiable advantages and risks, and explains how to use ClF3 safely and efficiently for in-situ cleaning of CVD chambers.
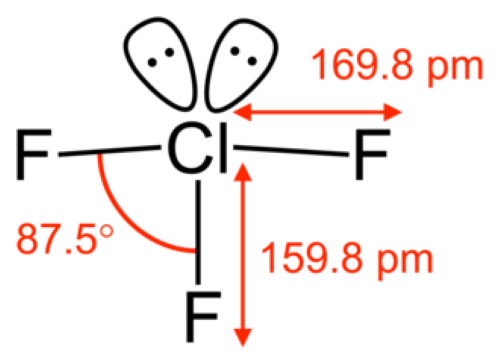 [1]
[1]
Figure 1. Chlorine trifluoride
Understanding In-Situ Cleaning and Its Importance for Maintaining CVD Efficiency
Chemical Vapour Deposition (CVD) is a process used to deposit thin layers on substrates. Deposits from by-products, such as carbon residues, metal remnants and other compounds, accumulate on chamber surfaces during semiconductor manufacturing. If these deposits are not removed, they may reduce semiconductor yield and affect device performance. In-situ cleaning maintains chamber efficiency and reduces process downtime, as cleaning is conducted without removing the chamber from the production line.
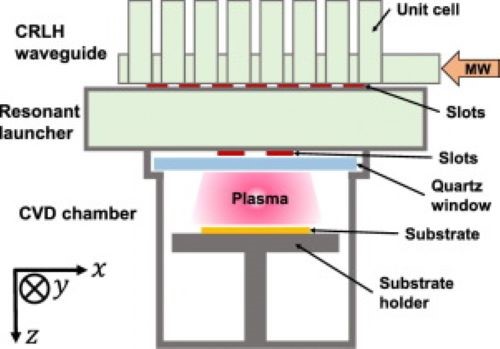 [2]
[2]
Figure 2. CVD Chamber
A typical in-situ cleaning process includes the following steps:
1. Removal of Deposits: The primary objective is to remove deposits that accumulate during semiconductor manufacturing. Such deposits include process by-products, native oxides, metal fluorides and organic residues.
2. Maintaining Chamber Functionality: Cleaning preserves the operational capacity of the CVD chamber. It also reduces defects and improves yield. The process is performed without removing the chamber from production, thereby minimising downtime.
3. Use of Cleaning Agents: Cleaning agents are selected based on chamber materials and deposit types. ClF3 is used because it removes deposits effectively.
Advantages and Disadvantages of Chlorine Trifluoride as a Cleaning Gas
Chlorine trifluoride is employed to maintain equipment cleanliness and performance. Its quantifiable advantages include:
Effectiveness: It removes deposits and leaves minimal residue. This property is essential in semiconductor manufacturing, where small deposits may reduce circuit performance.
Selectivity: The cleaning mechanism targets specific deposits without damaging the underlying substrate. This selectivity is critical for precise semiconductor fabrication.
Versatility: ClF3 is effective at removing various deposits including native oxides, metal fluorides and organic compounds, thereby preserving the condition of CVD chambers.
Chlorine trifluoride is therefore used in the semiconductor industry to maintain equipment function and reduce defects during production.
The use of ClF3 presents significant disadvantages:
Toxicity: ClF3 is highly toxic. Its handling requires strict adherence to safety protocols regarding usage and storage.
Reactivity: ClF3 reacts with moisture, air and numerous organic substances. This reactivity may lead to fires or explosions if not managed properly.
Specialised Handling: Due to its hazardous nature, ClF3 requires specialised equipment and procedures, which may increase operational costs and process complexity.
Environmental Considerations: Owing to its reactivity and toxicity, ClF3 poses environmental risks. Its use is subject to strict environmental and safety regulations.
Safety Aspects When Handling and Storing Chlorine Trifluoride in Semiconductor Cleaning
Strict safety protocols govern the handling and storage of ClF3 in the semiconductor industry. The gas must be stored in a cool, dry location that is free from moisture and heat sources. It is transported and stored in specially designed containers that can withstand its corrosive behaviour. When working with ClF3, personnel are required to wear respiratory masks, gloves and protective clothing.
Conclusion
In summary, chlorine trifluoride is a cleaning gas with quantifiable advantages and measurable risks. The semiconductor industry must adhere to strict safety procedures during its handling and storage. For further information, please visit our homepage.
Reference:
[1] Chlorine trifluoride. (23/08/2023). In Wikipedia. https://www.wikidata.org/wiki/Q411305
[2] Justas Zalieckas, Paulius Pobedinskas, Martin Møller Greve, Kristoffer Eikehaug, Ken Haenen, Bodil Holst, Large area microwave plasma CVD of diamond using composite right/left-handed materials, Diamond and Related Materials, Volume 116, 2021, 108394, ISSN 0925-9635, https://doi.org/10.1016/j.diamond.2021.108394.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Chin Trento
Chin Trento



