Cerium(IV) Ammonium Nitrate: A Commonly Used Oxidant
Cerium(IV)-ammonium nitrate (CAN) is a frequently used oxidising agent. The molecular formula of Cerium(IV)-ammonium nitrate is Ce(NH4)2(NO3)6. It is an orange-red crystal, soluble in water and ethanol, nearly insoluble in concentrated nitric acid and subject to decomposition in air. It is commonly employed as an oxidising agent in the corrosion of circuits and in the production of other cerium compounds.
%20Ammonium%20Nitrate.jpg)
Cer(IV)-ammonium nitrate
Cerium(IV)-ammonium nitrate is a potent oxidising agent. Under acidic conditions it exhibits a higher oxidising capacity than F2, XeO3, Ag2+, O3 or HN3. In aqueous solutions and other protic solvents it functions as a one‑electron oxidant. Its consumption is indicated by a colour change from orange to light yellow.
Owing to its limited solubility in organic solvents, reactions incorporating Cerium(IV)-ammonium nitrate are typically performed in mixed solvents such as water/acetonitrile. In the presence of other oxidising agents, for instance sodium bromate, tert‑butyl hydroperoxide and oxygen, Ce4+ is recycled, thereby enabling a catalytic reaction. Furthermore, it is used as an effective nitrating reagent.
CAN exerts an oxidising effect on oxygenated compounds including alcohols, phenols and ethers, and it displays particular activity towards secondary alcohols. For instance, it oxidises benzyl alcohol to yield the corresponding aldehydes and ketones (Equation 1). p‑Nitrobenzyl alcohol is oxidised by the catalytic oxidation system CAN/O₂ to produce p‑nitrobenzyl ketone. Moreover, for specific secondary alcohols such as 4‑enol or 5‑enol, cyclic ether compounds are obtained under the influence of CAN (Equation 2).
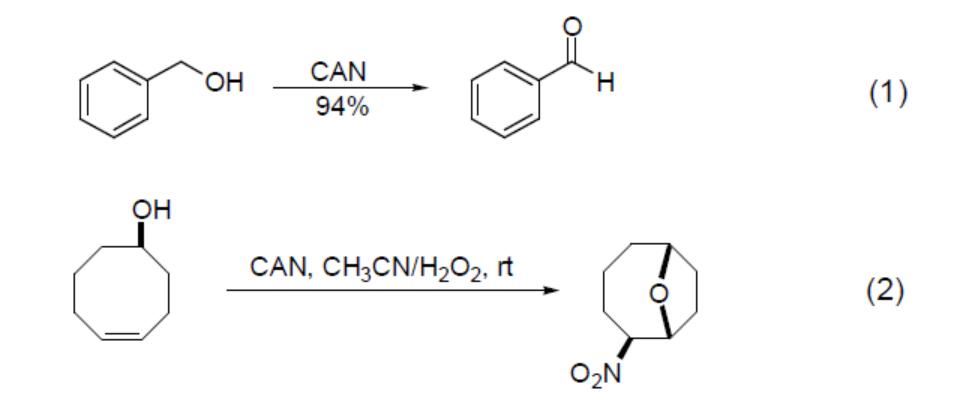
Catechol, hydroquinone and their methyl ether derivatives are oxidised by CAN to yield quinones. This is demonstrated by the conversion of catechol to o‑benzoquinone (Equation 3), the rapid oxidation of hydroquinone to p‑benzoquinone (Equation 4) under the action of CAN and ultrasound, and the transformation of aryl ethers to p‑benzoquinone.
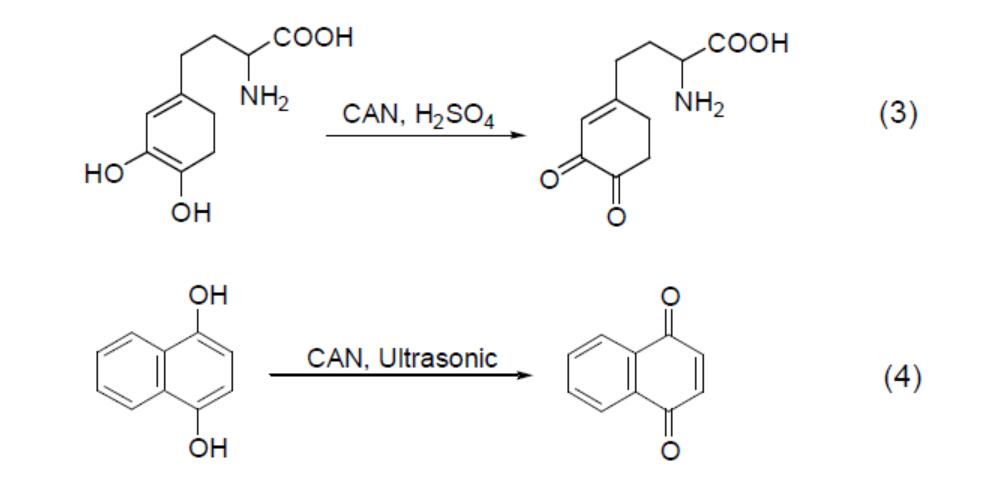
The oxidation of epoxy compounds by CAN can also yield dicarbonyl compounds (Equation 5). Furthermore, CAN oxidises carbonyl compounds with specific structures; for example, it converts polycyclic cage ketones to lactones (Equation 6).
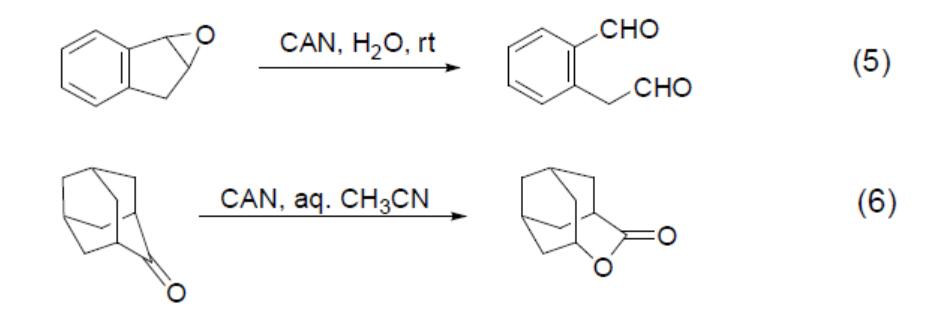
As a one‑electron oxidant, CAN facilitates both intermolecular and intramolecular carbon–carbon bond formation. For example, the oxidative addition reaction of a 1,3‑dicarbonyl compound with a styrene system occurs under the influence of CAN (Equation 7) and the dimerisation of aniline itself takes place (Equation 8).
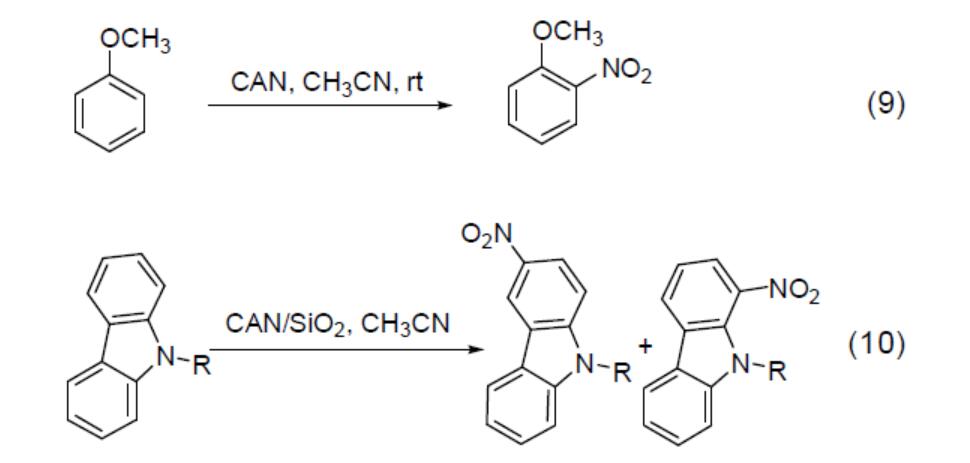
In addition to oxidation reactions, CAN is used as a nitrating reagent for aromatic systems. In acetonitrile, CAN reacts with anisole to yield ortho‑nitrated products (Equation 9). Given its strong oxidising properties, polynitration reactions of aromatic rings are frequently observed, which may lead to the formation of polymers that are difficult to separate. Studies have demonstrated that the adsorption of CAN onto silica gel reduces its oxidising behaviour, thereby diminishing the formation of polynitro products. For instance, in acetonitrile when silica gel is employed as a carrier in the nitration of carbazole and 9‑alkylcarbazole with CAN, the yield increases to 70–80% (Equation 10).
Conclusion
We thank you for reading our article and hope it has contributed to an enhanced understanding of the frequently used oxidising agent Cerium(IV)-ammonium nitrate. Should you wish to learn more about Cerium(IV)-ammonium nitrate and other powders, please visit Stanford Advanced Materials (SAM) for further information.
As a global supplier of Cerium(IV)-ammonium nitrate products, Stanford Advanced Materials (SAM) has more than two decades of experience in the manufacture and sale of Cerium(IV)-ammonium nitrate. It provides quality Cerium(IV)-ammonium nitrate to meet the research, development and production requirements of its customers. Consequently, we are confident that SAM will be your preferred Cerium(IV)-ammonium nitrate supplier and business partner.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us





 Chin Trento
Chin Trento


