Comparative Insights Into Precious Metal Catalysts: Powder Vs. Pellet Forms
1 Introduction
Precious metal catalysts are essential for many chemical industry reactions due to their specific electronic structure and chemical stability. They deliver performance in terms of selectivity, cooperative effect and stability, and they play a key role in two important industrial reactors: kettle reactors and fixed‐bed reactors. The particle size and shape of precious metal catalysts have a significant impact on reaction efficiency and product selectivity. Consequently, understanding and optimising these parameters is imperative for achieving efficient catalytic processes.
2 Characteristics of Precious Metal Catalysts
Precious metal atoms possess d‐electrons in their outermost layers, which impart notable reactivity. This property allows the formation of covalent bonds between oxygen and hydrogen atoms, thereby facilitating oxidation and reduction processes under lower energy barriers. Accordingly, single atoms, metal oxides and metal complexes can be employed as catalysts. Precious metal catalysts are used for their ability to direct product formation, generate a cooperative effect and maintain stable performance.
1. Selectivity: Catalysed reactions generally follow multiple reaction pathways that lead to various products. The selectivity of the catalyst influences the energy barriers of these pathways and determines the principal products and their relative quantities under specific reaction conditions. Different precious metal catalysts for the same reaction yield varying product distributions; the same catalyst may facilitate different reactions under different conditions.
2. Cooperative Effect: Precious metal catalysts may be applied in combination. When utilised together, the overall catalytic activity may be increased. In addition, precious metals and other metals can form binary or multiple alloys with varying morphologies and ratios. This mixture reduces the quantity of precious metals required and enhances both the selectivity and duration of the catalytic reaction. When combined with various supports, the catalytic performance differs significantly according to the preparation method. Given these factors, the range of applications and research areas is broad.
3. Stability: Precious metals are inherently chemically stable; they resist oxidation and are not easily attacked by common acids or bases. They also have high melting points and good thermal stability, and their properties remain consistent under most reaction conditions. Precious metals are not prone to the formation of halides or sulphides under normal conditions and are thus not readily contaminated. Although temporary deactivation may occur via adsorption of sulphur or CO, such effects can be reversed under specific conditions. However, the high stability of these catalysts also means that their elution and recovery are challenging.
4. Catalytic Activity: This property measures the efficiency of a catalyst. Generally, the catalytic activity of precious metal catalysts exceeds that of conventional catalysts. Their specific electronic structure and lattice morphology allow for the formation of highly active surface sites. These sites are capable of adsorbing and activating reactants, thereby lowering the energy barrier between them and accelerating the reaction rate. The combination of high catalytic activity, selectivity and stability makes these catalysts particularly effective in many chemical processes.
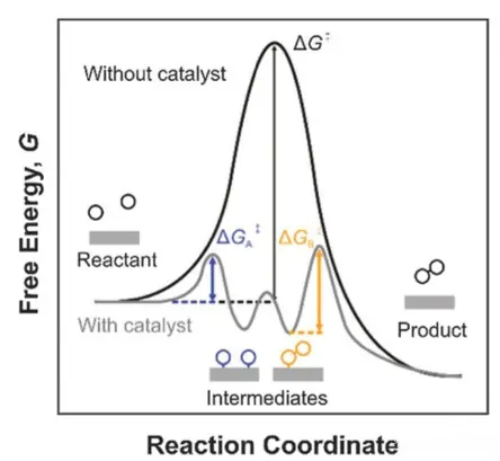
Fig. 1 Mechanism of Catalyst Action
3 Reactor Technologies: Kettle and Fixed‐Bed Systems
3.1 Kettle Reactors: Operation and Catalyst Utilisation
The kettle reactor, also known as a reaction vessel, is equipment used for conducting reaction processes. It is employed in single‐phase liquid reactions and in multiphase reactions (liquid–liquid, gas–liquid, liquid–solid, gas–liquid–solid, etc.). Typically, the reactor is large and processes substantial quantities of reactants. To ensure thorough contact between the reactants and the catalyst, the device is normally agitated mechanically or by air flow, sometimes using multi‐layered stirring paddles. The kettle reactor is designed to endure high temperatures and pressures. Temperature control is achieved by fitting a jacket to the reactor wall or by incorporating heat exchange surfaces within the device. External circulation may also be used to control and adjust the temperature.
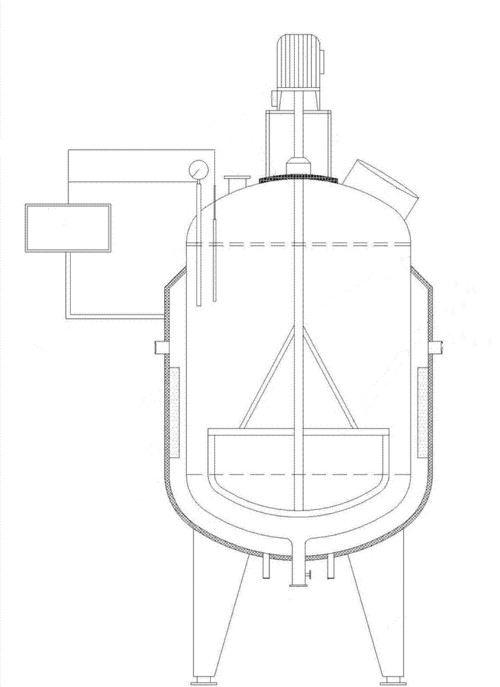
Fig. 2 Schematic of a Reaction Vessel
The reactor types are categorised into batch reactors and continuous reactors. In a batch reactor, raw materials are added all at once in a fixed ratio and removed once the reaction reaches a predetermined stage, whereas in a continuous reactor, raw materials are continuously fed and products are continuously removed.
Batch Reactor: Batch reactors offer operational flexibility and can be adapted easily to varying reaction conditions and product types. They suit small batches, multiple reaction types and longer reaction durations because there is no intermixture of materials during the process. However, they require additional steps for loading and unloading, and maintaining consistent product quality may be challenging.
Continuous Reactor: Continuous reactors yield consistent product quality and are simpler to operate and control. However, some degree of back-mixing may occur, which should be minimised by appropriate reactor design.
3.2 Fixed‐Bed Reactors: Performance and Catalyst Role
A fixed‐bed reactor is an apparatus filled with granular solid catalysts or solid reactants that form a packed bed. In these reactors, gas or liquid flows through the interstices of the stationary bed, resulting in an inhomogeneous reaction process. This type of reactor is considered a heterogeneous catalytic reactor. Because the solid particles remain fixed in place, there is a reduced mechanical loss of catalyst compared with those in moving or fluidised beds. Conversely, fixed‐bed reactors typically have poor heat transfer. In cases where the exothermic reaction heat is significant, temperature control within the reactor can become problematic. In fixed‐bed operation, catalyst replacement is not straightforward, and frequent regeneration may be necessary. Consequently, fluidised or moving bed reactors are sometimes used as alternatives.
There are three basic forms of fixed‐bed reactors. The first is the axial adiabatic fixed‐bed reactor, in which the liquid flows axially from top to bottom without heat exchange with the environment. The second form is the radial adiabatic fixed‐bed reactor, where the liquid flows radially (either centrifugally or centripetally) with no external heat exchange. Radial and axial reactors have shorter flow paths, larger cross‐sectional areas for the flow channel and lower pressure drops. However, the structure of the radial reactor is more complex. Both forms operate adiabatically, which is acceptable when the thermal effect of the reaction is limited or when the reaction system can tolerate temperature fluctuations. The third type is the tube fixed‐bed reactor, which consists of several parallel reaction tubes. In this arrangement, the catalyst is placed in or between the tubes while the heat transfer medium heats or cools either the tubes or the interior of the tubes. The tube diameter generally ranges between 25 and 50 mm, and the number of tubes can reach several tens of thousands. Tube fixed‐bed reactors are applicable to reactions with significant thermal effects. In addition, multi‐stage fixed‐bed reactors, in which reactors are combined in series with interposed heat exchangers or additional temperature regulating materials, are used when segmented temperature control is necessary to maintain optimal conditions.
4 Application of Precious Metal Catalysts in Reactor Technologies
4.1 Powder Form in Kettle Reactors
In chemical production, precious metal catalysts are employed because of their catalytic activity and selectivity in a wide range of reactions. In kettle reactors, these catalysts are commonly used in powder form. Their high specific surface area improves contact between reactants and catalyst, which accelerates the reaction rate. Highly dispersed precious metal powder catalysts are used in many organic synthesis reactions, such as hydrogenation, carbonylation and coupling reactions. These catalysts are prepared by mixing a precious metal precursor solution with a support, followed by reduction treatment. Researchers have also developed single‐atom catalysts that disperse individual metal atoms on supports with high specific surface areas. This method increases catalytic efficiency and lowers the consumption of precious metals. In liquid‐phase reactions, such as hydrogenation and oxidation, single‐atom catalysts exhibit performance that is comparable to or better than that of conventional nanoparticle catalysts.
A documented example is the contact process for the manufacture of sulphuric acid. In 1831, Phillips proposed a method that used platinum as a catalyst to accelerate the reaction between sulphur dioxide and oxygen, producing sulphur trioxide. This process was industrialised in 1875 under the supervision of the German chemist Maisel. The introduction of the contact process improved both the yield and purity of sulphuric acid. The process required complete contact between the reactants and the catalyst, an idea that later informed the development of multiphase catalysis in industrial applications.
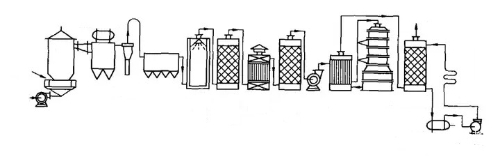
Fig. 4 Process Flow of the Modern Contact Method for Sulphuric Acid
4.2 Pellet Form in Fixed‐Bed Reactors
The synthesis of vinyl acetate via the gas‐phase oxidation of ethylene is performed in a fixed‐bed reactor system. Researchers have systematically analysed the active layers of precious metal oxidation catalysts and examined methods to control particle heterogeneity and optimise catalyst parameters for fixed‐bed reactors. This analysis indicates that modifying the shape and structure of catalyst particles can improve reaction conversion and product selectivity. The loaded Pd–Au catalyst is one of the most frequently employed catalysts for vinyl acetate synthesis via ethylene gas‐phase oxidation. To assess catalytic performance, researchers assemble fixed‐bed reactor systems and examine how different reaction conditions affect catalyst performance. For example, the Au/Pd ratio significantly influences conversion and selectivity. At an Au/Pd ratio of 0.86, the Pd–Au/4A catalyst achieved improved performance. The particle size of the catalyst is also critical for gas‐phase vinyl acetate synthesis. Catalyst supports with particle sizes of approximately 3–7 mm offer adequate mechanical strength and low pressure drop, facilitating catalyst bed packing and reaction operation. An optimal specific surface area ranging from 50 to 800 m²/g provides additional active sites, thereby enhancing catalytic efficacy.
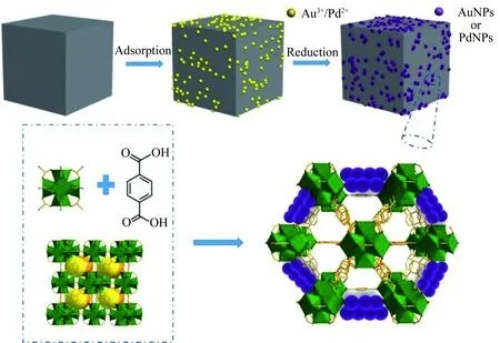
Fig. 5 Schematic Strategy for the Synthesis of Loaded Au–Pd Catalysts
4.3 Impact of Catalyst Particle Size on Application
In kettle reactors, catalysts must be uniformly dispersed to ensure sufficient contact between the reactants and the catalyst, thereby improving reaction efficiency. Powder form precious metal catalysts offer a larger surface area and consequently more active sites per unit volume. Kettle reactors typically operate for liquid‐phase or gas–liquid-phase reactions, and powder catalysts mix more easily with liquids or gases. Furthermore, kettle reactors are usually used in batch or semi-continuous processes. Under these conditions, the powder catalyst disperses uniformly in the reaction medium, which aids in controlling the reaction temperature and ensuring even heat distribution, thereby preventing local overheating.
In fixed‐bed reactors, the catalyst is typically immobilised on a support to form a catalyst bed. Pellet form catalysts are better suited for this type of reactor because they fill the bed effectively, maintain mechanical stability and exhibit favourable hydrodynamic properties. Fixed‐bed reactors are generally used in continuous processes, and the immobilised catalyst facilitates stable operation. With the catalyst fixed in place, reaction products can be recovered directly from the catalyst bed without additional separation steps. For reactions conducted under high pressure, catalyst particles can be compacted to reduce void spaces and thereby improve reaction conversion.
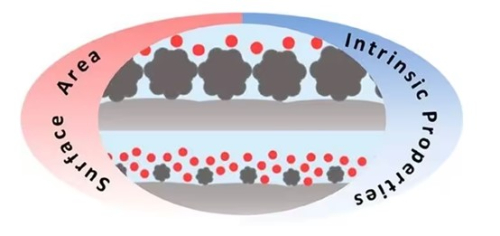
Fig. 6 Schematic Diagram of Catalyst Particle Contact for Different Particle Sizes
5 Conclusion
Precious metal catalysts are employed in chemical manufacturing due to their high catalytic activity, selectivity and thermal stability. Their use in both kettle and fixed‐bed reactors demonstrates a broad range of applications and underscores the importance of optimising reaction performance through careful catalyst design and reactor selection. In critical chemical processes, such as the gas‐phase oxidation of ethylene to vinyl acetate, the rational selection and configuration of precious metal catalysts is a decisive factor in improving reaction conversion and product quality. Moreover, the catalyst particle size and form directly affect contact efficiency and catalytic activity. Researchers and engineers must therefore control these parameters precisely to achieve optimal performance. Despite their many advantages, the recovery and recycling of precious metal catalysts remain challenging and further research and technological advances are required. Stanford Advanced Materials (SAM) provides a broad selection of high‐purity precious metal catalysts that can be customised upon request. Prospective clients are invited to browse the product list or to contact one of the SAM specialists for advice.
Related Readings:
Precious Metal Catalysts: A Closer Look at the Impact of Particle Size
Common Reaction Types of Homogeneous Precious Metal Catalysts
Precious Metal Catalysts for the Petroleum Sector
Advantages of Precious Metal Catalysts
References:
[1] Gordeeva A N, Shesterkina A A, Vikanova V K, et al. Naphthalene and its Derivatives – Hydrogenation for Hydrogen Storage: A Comparative Analysis of the Roles of Precious and Non-Precious Metal Catalysts – A Review [J]. International Journal of Hydrogen Energy, 2024, 69.
[2] Qi C X, Lang F, Li C, et al. Cooperative Effects of MOFs and Noble Metals in Photocatalytic Reactions: Mechanisms and Applications [J]. ChemPlusChem, 2024.
[3] Fairlie M A. Book Review: The Manufacture of Sulphuric Acid (Contact Process) [J]. Industrielle und technische Chemie, 2002, 18(1).
[4] Homme C A, Othmer F D. Optimised Conditions for Sulphuric Acid Manufacture in the Contact Process [J]. Industrie- und Ingenieurchemie, 2002, 53(12).

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us




 Chin Trento
Chin Trento


