What Is An All-aluminum Body?
When discussing a full-aluminium car body, its most well‐known feature is its low weight. Apart from high‐priced premium models that incorporate significant carbon fibre, lightweight vehicles generally use a high proportion of aluminium.
The concept of a full‐aluminium body means that the vehicle’s structure is primarily composed of an aluminium alloy. Non‐aluminium components may be included, and aluminium may also occur in the form of other alloys.
In fact, the term aluminium alloy does not indicate a single type but covers many varieties. The international standard distinguishes these alloys with a four‐digit number and one letter. For example, the construction industry frequently uses 6063‑T5. The first digit, 1, denotes aluminium that is over 99 % pure; digits 2 to 8 represent alloys incorporating copper, manganese, silicon, magnesium, zinc and other elements; and digit 9 refers to the readiness group. The subsequent numbers primarily serve as identifiers. Consequently, each aluminium alloy designation corresponds to its specific properties.
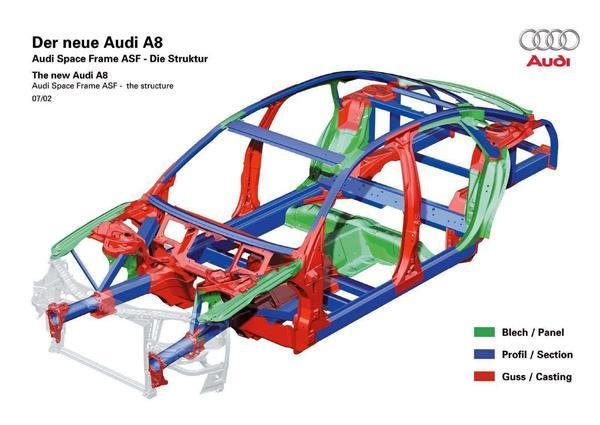
Currently, series 5 and 6 are used primarily for producing full‐aluminium car bodies. They feature low density, high tensile strength relative to their weight and satisfactory fatigue resistance. They are employed in the ASF full‐aluminium body by Audi. Additionally, series 2 and 7 have limited applications. The copper–aluminium alloy from series 2, which exhibits high hardness, is sometimes applied in sheet metal parts. Series 7 is utilised in aerospace and by military organisations given that it comprises ultrahard, corrosion‐resistant and wear‐resistant materials that incur a high cost.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us



 Chin Trento
Chin Trento


