How Can Nano Tungsten Disulfide Be Used Today?
Single-layer Nano-Wolframdisulfide exhibits two two-dimensional ordered layered structures and specific physical and chemical properties. Its anisotropic layered structure confers distinct optical, electronic and mechanical properties. How can Nano-Wolframdisulfide be utilised today? This article attempts to answer the question.

-
Biomedical Materials
Wolframdisulfide can be employed as a medical contrast agent because it is a layered two-dimensional material with a hexagonal crystal system that exhibits measurable absorption and biocompatibility in the near-infrared (NIR) spectrum.
Researchers have developed multifunctional two-dimensional Nano-Wolframdisulfide materials by labelling WS2 to determine its spatial distribution within cells and tissues. Combined with medical diagnostic, imaging and treatment techniques, it is used in the detection and analysis in biomedical research, cancer therapy, toxicology, cellular imaging, antibacterial applications and other areas.
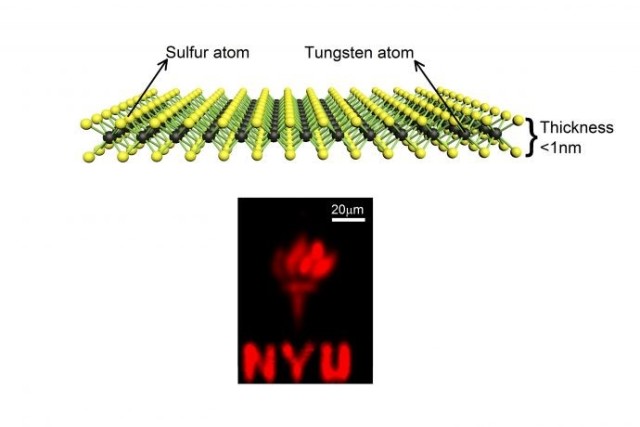
-
Mechanical Lubricants
Wolframdisulfide is a layered two-dimensional material with a hexagonal crystal structure. It exhibits weak interlayer interactions and low binding energy, which facilitate movement during frictional processes and result in a reduced friction coefficient.
Furthermore, Nano-Wolframdisulfide can adsorb and fill irregular surfaces over time, thereby contributing to an additional lubricating effect. Transition metal Nano WS2 provides effective lubrication across the temperature range of -273 ℃ to 425 ℃. Owing to its capacity to function under harsh conditions, Wolframdisulfide is used in military, aerospace, satellite, space vehicle and other advanced technological applications.
-
Catalysis
Wolframdisulfide is an indirect semiconductor with a high specific surface area. In a similar manner to Wolframoxid, it functions as a photocatalyst by absorbing visible light to generate electrons and holes. The holes react with water to produce hydroxyl radicals (-OH), which oxidise large organic molecules into smaller organic molecules and inorganic ions.
Wolframdisulfide is also applicable in the petrochemical industry as a tungsten heteropoly catalyst. It has been observed to display high cracking efficacy, consistent catalytic activity, an extended operational duration, low toxicity, thermal stability and chemical stability. Consequently, it can serve as an effective hydro-treating catalyst for both hydrocracking and hydrodesulphurisation in oilfield applications.
-
New Energy
Within the field of new energy, Wolframdisulfide is regarded as a material of significant interest. Its structure closely resembles that of graphene and comprises a lamellar configuration connected by weak van der Waals forces. The material is generally within the nanometre scale. Interlayer cavities and gaps can be utilised for the storage of hydrogen and Lithium.
Wolframdisulfide demonstrates adequate thermal stability and supports repeated reuse with cyclic charge–discharge operations. Consequently, it is appropriate for energy storage applications including solar cells, fuel cell anodes, Lithium battery anodes and supercapacitors.
Conclusion
We appreciate your review of this article and trust that it clarifies current applications of Nano-Wolframdisulfide. For further information on Nano-Wolframdisulfide, please visit Stanford Advanced Materials.
Stanford Advanced Materials (SAM) is an international supplier of tungsten products. With over two decades of manufacturing and commercial experience, the company provides Nano-Wolframdisulfide designed to fulfil research and production requirements. Consequently, SAM is positioned to be a reliable Nano-Wolframdisulfide supplier and collaborator.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us




 Chin Trento
Chin Trento


