Case Study: Enhancing Fuel Cell Performance With Platinized Titanium Anodes
Introduction
Platinized Titanium Anodes are employed in fuel cell applications because of their electrocatalytic activity and corrosion resistance. They play a vital role in fuel cell systems by enabling energy conversion through electrochemical reactions. This article explains how platinized titanium anodes are used in fuel cells.
 [1]
[1]
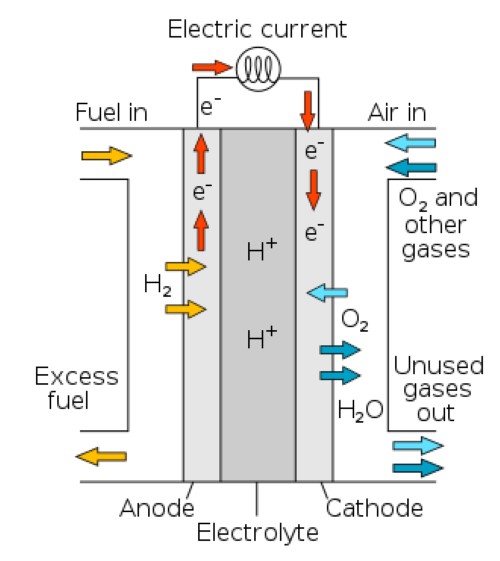
Figure 1. Diagram of a proton-conducting fuel cell
Advantages of Platinized Titanium Anodes
Platinized titanium anodes are specific components used in fuel cells that require efficient electrochemical processes. They are manufactured by depositing a platinum coating on a titanium substrate. This process produces a hybrid material that utilises the distinct properties of platinum and titanium.
Figure 2. Platinized Titanium Anodes
This anode configuration offers several advantages in fuel cell technology.
Increased electrocatalytic activity: Platinum acts as a catalyst that accelerates fuel cell reactions. Coating a titanium substrate with platinum improves the electrochemical reaction rate.
Corrosion resistance: Titanium is chosen for its inherent corrosion resistance. This property ensures the electrode remains stable in the chemically active environment of a fuel cell over prolonged periods.
Cost efficiency and platinum utilisation: Platinum is an expensive metal. Using a titanium substrate reduces the amount of platinum required and lowers overall costs. This is important for scaling fuel cell production for commercial applications.
Longevity and durability: The combination of titanium’s durability and platinum’s catalytic function results in an anode with an extended operational life. This longevity is essential for practical and cost-effective fuel cell systems.
Fuel Cell Applications of Platinized Titanium Anodes
Due to these properties, platinized titanium anodes are used in various fuel cells, including proton exchange membrane fuel cells (PEMFCs) and solid oxide fuel cells (SOFCs). They contribute to the electrochemical reactions necessary for energy conversion.
PEMFCs: In PEMFCs, the anode catalyses the oxidation of hydrogen. It splits hydrogen into protons and electrons. The protons migrate through the proton exchange membrane, and the electrons travel via an external circuit to generate electricity. The platinum layer increases the reaction rate, thereby enhancing the cell’s performance.
SOFCs: Platinized titanium anodes are also applied in SOFCs, which operate at higher temperatures than PEMFCs. In SOFCs, the anode catalyses the oxidation of hydrogen or hydrocarbon fuels. It enables the splitting of hydrogen molecules and the release of electrons that travel through an external circuit to produce electricity. The high operating temperatures assist the electrocatalytic process, and the platinum surface supports effective fuel oxidation under these conditions.
Conclusion
In conclusion, platinized titanium anodes play an important role in fuel cell applications by providing electrocatalysis, durability, corrosion resistance, and versatility in handling various fuels. Their contribution to the fundamental electrochemical reactions in fuel cells supports progress in clean energy conversion and sustainable electricity generation. Stanford Advanced Materials (SAM) offers various anodes, including platinized titanium anodes and platinized niobium mesh anodes. For further information, please visit our homepage.
Reference:
[1] Fuel Cell. (2023, 14/08/2023). In Wikipedia. https://en.wikipedia.org/wiki/Fuel_cell

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Chin Trento
Chin Trento


