Essential Electronic Materials: Part 6 - Conductive And Insulating Materials
1 Introduction
In electronic materials, differentiating between conducting materials and insulating materials is essential for the proper operation of electrical and electronic devices. Conducting materials such as metals, alloys, conductive ceramics and superconductors are required for efficient energy transfer, high-speed calculations and energy storage. These materials permit controlled electron movement and are indispensable for operating electrical circuits and devices. Conversely, insulating materials provide necessary safety and stability by preventing unwanted current flow and protecting electronic components from external influences. This section presents the fundamental properties, applications and technical developments of conducting and insulating materials, and it describes their roles in modern technology.
2 Conductive Materials
2.1 Metals and Alloys
The high electrical conductivity of metals and alloys results from their specific structural properties. Due to the low ionisation energy of metal atoms, valence electrons can detach easily and become free electrons. These electrons move throughout the metal’s crystal lattice and conduct electrical current efficiently. Metal atoms are linked by metallic bonds and arrange in dense crystal structures such as body-centred cubic, face-centred cubic and hexagonal close-packed forms. This arrangement permits the formation of a continuous sea of electrons. The electron cloud increases both electrical and thermal conductivity. In alloys, additional elements are introduced into the base metal. This action produces homogeneous or inhomogeneous solid solutions or compounds. Processes such as solid solution strengthening and precipitation hardening adjust the electrical conductivity while increasing mechanical strength and corrosion resistance, thereby enabling use in complex environments and under specific technical requirements.
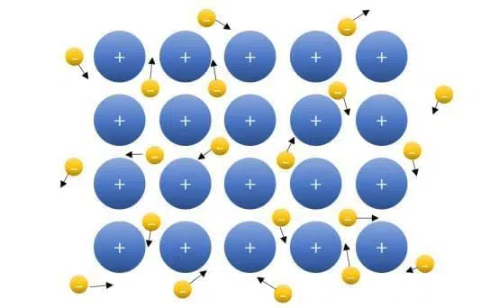
Fig. 1 Free electrons move through the lattice structure of a metal
Metals and alloys have an extensive range of properties as conducting materials. Metallic conductors such as copper and silver exhibit extremely high conductivity owing to high electron density and low resistivity. Alloys generally have slightly lower conductivity than pure metals due to electron scattering caused by dopant atoms; their performance may be improved by optimising composition. Furthermore, the thermal conductivity of metals is attributable to the efficient heat transfer by free electrons. Materials with high thermal conductivity, for example copper, are frequently used for heat dissipation. Aluminium alloys, for example, display higher tensile strength and durability compared to pure aluminium and are employed in aerospace and power transmission. Some alloys (for example, brass and stainless steel) form surface oxide layers that provide good corrosion resistance. Metals with high melting points such as tungsten and molybdenum and their alloys maintain their conductivity and structural stability at high temperatures. Consequently, they find application in extreme environments within electrical and electronic devices.
Fig. 2 Various metal wires
2.2 Conductive Ceramics
The conductivity of conductive ceramics is based on their specific crystal structure and electron transport mechanism. Some ceramics achieve conductivity through ion migration (for example, zirconium oxide), while others use electron transport (for example, titanium oxide). Doping with selected metals or oxides (for example, calcium-doped zirconium oxide or tin-doped indium oxide) increases the concentration of free charge carriers. Moreover, polycrystalline conductive ceramics may have defects at grain boundaries that affect conduction paths, but their conductivity and mechanics can be improved by optimising high-temperature sintering processes.
Conductive ceramics combine high temperature resistance with the ability to conduct electrical current. Their performance varies from semiconducting to good conductors, and specific performance depends on composition and the degree of doping. These ceramics maintain stable conductivity at high temperatures and function under extreme conditions. They also offer high corrosion resistance in acidic and alkaline conditions compared to metals. Despite inherent brittleness, their high hardness and compressive strength allow them to withstand mechanical stress. Transparent conductive ceramics such as indium tin oxide (ITO) are used in optoelectronic devices.
Conductive ceramics are used in electronics, energy and sensor applications. In electronics and optoelectronics, indium tin oxide (ITO) serves as a transparent electrode in touchscreens, LCDs and OLED displays, while titanium oxide is utilised in photovoltaic cells, photocatalytic devices and sensors. In the energy sector, calcium-doped zirconium dioxide (CaZrO3) functions as an electrolyte in solid oxide fuel cells, whereas zinc oxide is applied in varistors and transparent conducting films. For high temperature and extreme conditions, silicon carbide (SiC) and silicon nitride (Si3N4) are used for high temperature electronics, high-frequency devices and aerospace components. Conductive ceramics are further used in gas sensors and thermistors, and antistatic coatings based on ceramic powders provide static protection in electronic devices.
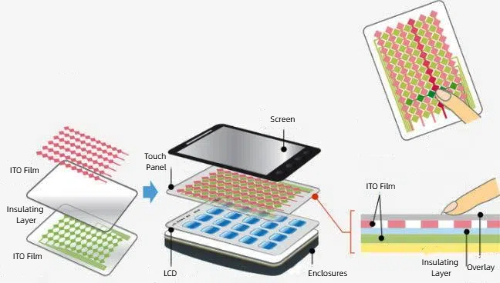
Fig. 3 ITO film for touch panels
2.3 Conductive Glass
Conductive glass generally comprises a highly transparent glass substrate bonded to a conductive surface coating. The electrical conductivity mainly originates from a transparent conductive oxide (TCO) layer covering the surface. The glass substrate is usually made of soda-lime or quartz glass, which offers high mechanical strength and good optical properties. The conductive layer is formed from common materials such as indium tin oxide (ITO), fluorine-doped tin oxide (FTO) and aluminium-doped zinc oxide (AZO). These coatings are applied by methods such as vacuum sputtering or chemical vapour deposition (CVD), and typically have thicknesses between ten and one hundred nanometres. Doping the oxides with elements such as tin, aluminium or fluorine increases the charge carrier concentration, thereby enhancing conductivity.
Conductive glass combines optical transparency with electrical conductivity and offers a number of favourable properties. The conductive coating typically achieves a light transmittance of 80% or more, while maintaining low reflectance. The specific resistance of the coating is generally between 10-3 and 10-4 Ω-cm, which meets the requirements of most electronic devices. The glass substrate provides high mechanical strength and thermal resistance. The coating is bonded securely and can tolerate elevated temperatures. In addition, conductive glass is resistant to oxidation and corrosion, which supports long-term use in environmental conditions. Recently, research has focused on flexible conductive glass deposited on polymer or ultra-thin glass substrates, thereby expanding potential applications.
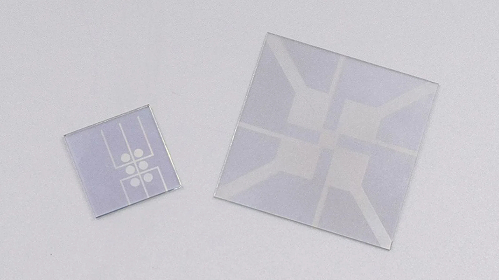
Fig. 4 Conductive ITO glass
2.4 Superconducting Materials
Superconducting materials are a class of substances that exhibit zero electrical resistance below a specified temperature and expel magnetic fields completely. They are categorised according to their critical temperature and structural properties. Low-temperature superconductors (LTS) such as niobium (Nb), niobium-titanium alloys (Nb-Ti) and niobium-tin (Nb3Sn) have critical temperatures typically below 30 K. They require cooling with liquid helium or liquid nitrogen and are used in devices such as magnetic resonance imaging (MRI) systems and particle accelerators. High-temperature superconductors (HTS) such as yttrium-barium-copper-oxide (YBCO) and bismuth-strontium-calcium-copper-oxide (BSCCO) have critical temperatures above 77 K. They can be cooled with liquid nitrogen, which lowers operating costs, and are applicable for power transmission and magnetic levitation at high temperatures. Iron-based superconductors, including compounds such as iron selenide (FeSe) and iron arsenide (LaFeAsOx)₋xFx, offer structural stability and significant magnetic field exclusion. Organic superconductors such as fullerenes (C60) or aromatic compounds present flexibility and low weight, making them suitable for flexible electronic devices. Topological superconductors that combine superconductivity with topological properties are under investigation for applications in quantum computing and electronic systems.
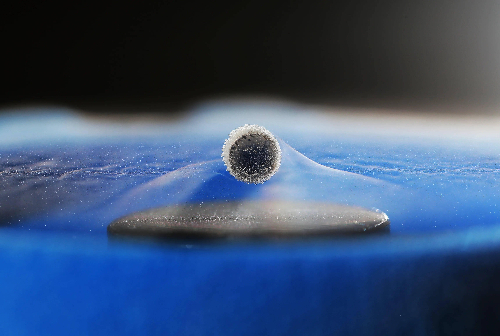
Fig. 5 Superconducting material
Superconducting materials display specific characteristics in electrical applications. Their zero resistance permits current to flow without energy loss. This property reduces energy consumption during long-distance transmission and high-efficiency energy storage. Superconductors also exhibit complete magnetic field expulsion (the Meissner effect). This effect is used to maintain stable levitation in magnetic levitation systems and frictionless bearings. The critical temperature (Tc) defines the temperature at which a material transitions to the superconducting state. This temperature varies between materials. In low-temperature superconductors, cooling with liquid helium is required, whereas high-temperature superconductors can be cooled with liquid nitrogen, thereby reducing operational costs. The critical magnetic field (Hc) and critical current density (Jc) constrain the performance of superconductivity. When external magnetic fields or current densities exceed these limits, the superconducting state is lost. Materials with high critical parameters are preferred for environments with strong magnetic fields and high current loads, such as MRI scanners and particle accelerators. In addition, superconductors display the Josephson effect, where a tunnelling current flows between superconductors across an insulating barrier. This phenomenon finds use in extremely sensitive magnetic sensors, superconducting quantum interference devices (SQUIDs) and quantum computing. These properties provide superconducting materials with potential for efficient energy transmission, applications within strong magnetic fields and advanced technology.
Superconducting materials are applied in a broad range of fields. In energy and power systems, superconducting cables use zero resistance to enable transmission over long distances with reduced energy loss; superconducting generators offer increased energy efficiency and a reduction in size and weight; and superconducting magnetic energy storage (SMES) systems can rapidly store and release large quantities of energy for grid regulation and stabilisation. In medical and scientific research, low-temperature superconductors generate strong magnetic fields in MRI systems, while superconducting magnets operate in particle accelerators. Superconducting quantum interference devices (SQUIDs) are used in magnetoencephalography and geomagnetic surveys as highly sensitive magnetic sensors. In transport and engineering, superconducting magnetic levitation systems and high-temperature magnetic bearings are implemented in aerospace and industrial machinery. In information technology, superconducting materials are integral to quantum computers. Superconducting quantum bits based on the Josephson effect have driven research in this domain. Superconducting electronic components, such as filters and high-frequency amplifiers, are also used in communication and signal processing. In military and aerospace applications, superconducting electromagnetic systems are employed for controlled acceleration, while superconducting radars enhance signal detection and accuracy.
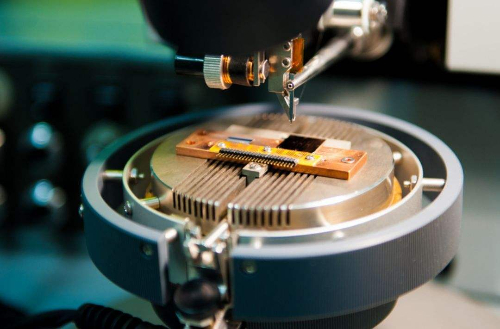
Fig. 6 Applications of superconducting materials
3 Insulating Materials
3.1 Inorganic Insulators
Inorganic insulators are substances with high electrical resistance and good thermal endurance. They are widely used for electrical insulation. Typical inorganic insulators include glass, ceramics and mica. Glass primarily consists of silicates (SiO2) whose structure is formed by covalently bound silica-oxygen tetrahedra. This structure restricts the movement of free electrons. Ceramics are usually made from materials such as aluminium oxide (Al2O3) and zirconium oxide (ZrO2), which create dense crystalline structures with very low electron mobility and ionic conductivity. Mica has a layered silicate structure with weak interlayer bonds that facilitate the production of thin sheets while providing good electrical insulation and thermal stability.
Inorganic insulators possess several advantageous characteristics. They exhibit a high specific resistance, generally above 1012 Ω-cm, which prevents leakage currents and ensures the safe operation of electrical devices. They also have high thermal resistance and can withstand temperatures of several hundred to thousands of degrees Celsius. Their mechanical strength enables them to endure significant mechanical loads, while mica sheets offer flexibility and can be processed into various forms. Inorganic insulators are chemically stable and resist erosion from acids, alkalis and moisture. Moreover, their excellent dielectric properties and high breakdown strength allow them to withstand high voltages without electrical breakdown, thereby ensuring safety in high-voltage applications.
Glass is commonly used as a high-voltage insulator in electrical devices and in the casings of vacuum circuit breakers, as well as an encapsulation material to protect electronic components. Ceramics are frequently employed as insulators in transformers and switchgear, as they provide both good dielectric performance and heat dissipation. They are further used as substrates in high-frequency devices and in the manufacture of insulators for spark plugs and high-voltage line components. Mica is often used as an insulating sheet in motors and generators that operate under high temperatures and pressures, and it is also utilised as an insulation material in high-frequency capacitors due to its low dielectric loss.

Fig. 7 Inorganic mineral insulators
3.2 Polymer Insulators
Polyvinyl chloride (PVC) is a polymer formed by the polymerisation of vinyl chloride monomers. It consists of linear or branched carbon-chlorine chains and exhibits high chemical stability. PVC has good electrical insulation and high electrical resistance, which prevents current leakage. It is highly resistant to chemicals such as acids, alkalis and salts, and also shows good abrasion resistance. Its thermal endurance is moderate, typically ranging from -10°C to 60°C. PVC is commonly used for the outer insulation of cables and wires and for protecting electrical devices, particularly in low-voltage applications.
Polyimide (PI) is a polymer with a rigid ring structure. Its main chain comprises imide groups (-C=O-N-) and it offers high mechanical strength and thermal resistance. Polyimides perform reliably at elevated temperatures, often above 250°C, and are therefore suitable for high-voltage and high-frequency devices. They also exhibit good mechanical strength, abrasion resistance and excellent chemical resistance against many solvents. Common applications include high-temperature cables, components in aerospace electrical systems, printed circuit boards (PCBs) and insulation for electronic components.
Polytetrafluoroethylene (PTFE) is a linear polymer formed by the polymerisation of tetrafluoroethylene monomers. The high electronegativity of the fluorine atom results in extremely low friction coefficients and excellent chemical stability. PTFE has a very low dielectric constant and provides outstanding electrical insulation, enabling its use in high-frequency and high-voltage contexts. It exhibits high chemical resistance against most chemicals, including strong acids, bases and solvents. It also remains effective over the temperature range of -200°C to 260°C, and it shows excellent abrasion resistance and low friction. Common applications include high-voltage cables, insulation for electronic components, linings for chemical pipelines and insulation where extreme conditions prevail.
Fig. 8 Plastics used as wire packaging
4 Conclusion
The materials discussed for conducting and insulating purposes perform complementary yet distinct roles in the design and function of electronic devices. Conducting materials, from metals such as copper and silver to superconductors, provide high electrical conductivity, mechanical strength and effective heat management. In contrast, insulating materials such as inorganic substances (e.g. ceramics) and polymers (e.g. PTFE) deliver essential electrical isolation, thermal endurance and mechanical strength. These materials ensure the protection, efficiency and longevity of electronic systems. Advances in materials science will continue to refine the performance of these substances and support progress in sectors including energy, communications, healthcare and aerospace.
Stanford Advanced Materials (SAM) is a leading supplier of high-quality conducting and insulating materials. The company provides reliable material solutions for critical applications.
Further reading:
Essential Electronic Materials: Part 1 – Silicon
Essential Electronic Materials: Part 2 – Silicon Carbide
Essential Electronic Materials: Part 3 – Germanium
Essential Electronic Materials: Part 4 – Gallium Compounds
Essential Electronic Materials: Part 5 – Carbon-Based Materials

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us





 Chin Trento
Chin Trento


